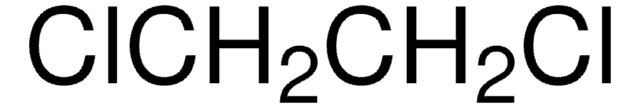D62209
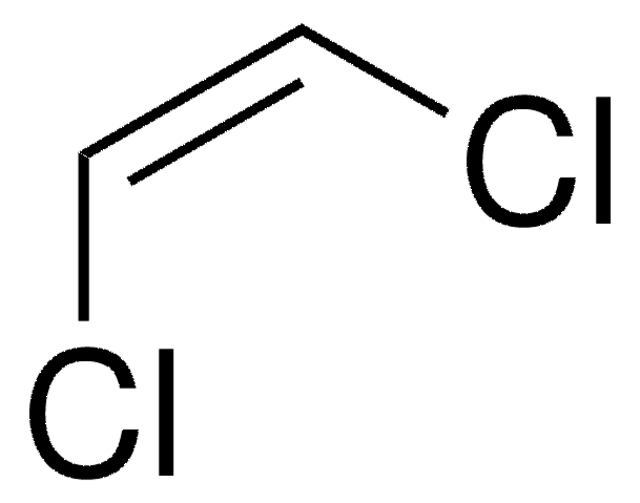
trans-1,2-Dichloroethylene
98%
Synonym(s):
trans-1,2-Dichloroethene, trans-Acetylene dichloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClCH=CHCl
CAS Number:
Molecular Weight:
96.94
Beilstein:
1420761
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
5.16 psi ( 20 °C)
Quality Level
Assay
98%
form
liquid
expl. lim.
12.8 %
refractive index
n20/D 1.446 (lit.)
bp
48 °C (lit.)
mp
−50 °C (lit.)
density
1.257 g/mL at 25 °C (lit.)
SMILES string
Cl\C=C\Cl
InChI
1S/C2H2Cl2/c3-1-2-4/h1-2H/b2-1+
InChI key
KFUSEUYYWQURPO-OWOJBTEDSA-N
Looking for similar products? Visit Product Comparison Guide
General description
trans-1,2-Dichloroethylene is an organic solvent used in freon cleaning agents.
Application
trans-1,2-Dichloroethylene can be involved in the synthesis of:
- (E)-Chloroenynes by reacting with 1-alkyne and piperidine in the presence of PdCl2(PPh3)2 catalyst in ethyl ether as a solvent.
- (E)-3-trimethylsilyl-1-chloro-1-propene by reacting with trimethylsilylmethylmagnesium chloride (TMSCH2MgCl) in the presence of Co(II) or Co(III) acetylacetonate.
- trans-1,2-Bis(tri-n-butylstannyl)ethylene which is an important reagent for the synthesis of myriad of natural products.
- trans-1-Chloroalkenes by reacting with Grignard reagents in the presence of tetrakis(triphenylphosphine)nickel as catalyst.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Chronic 3 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
42.8 °F - closed cup
Flash Point(C)
6.0 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Suzuki Coupling Reactions of (E)-and (Z)-Chloroenynes with Boronic Acids: Versatile Access to Functionalized 1, 3-Enynes
Tikad A, et al.
European Journal of Organic Chemistry, 2010(4), 725-731 (2010)
Cobalt-catalyzed mono-coupling of R3SiCH2MgCl with 1, 2-dihalogenoethylene: a general route to γ-substituted (E)-allylsilanes.
Kamachi T, et al.
Tetrahedron Letters, 45(24), 4677-4679 (2004)
Preparation of trans-1, 2-bis(tributylstannyl) ethylene.
Corey EJ and Wollenberg RH
The Journal of Organic Chemistry, 40(25), 3788-3789 (1975)
A convenient preparation of trans(or cis)-1-chloroalkenes from trans(or cis)-1, 2-dichloroethylene: A new synthesis of the sex pheromone of lobesia botrana.
Ratovelomanana V and Linstrumelle G
Tetrahedron Letters, 22(4), 315-318 (1981)
Florian Schevenels et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(13), 4335-4343 (2013-01-22)
Highly functionalised benzofurans have been prepared from ortho-hydroxyphenones and 1,1-dichloroethylene. The key intermediate, a chloromethylene furan, smoothly rearranged into the corresponding benzofuran carbaldehyde under acidic conditions. Some mechanistic investigations have been performed and several biologically active benzofurans have been synthesised.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service