D39002
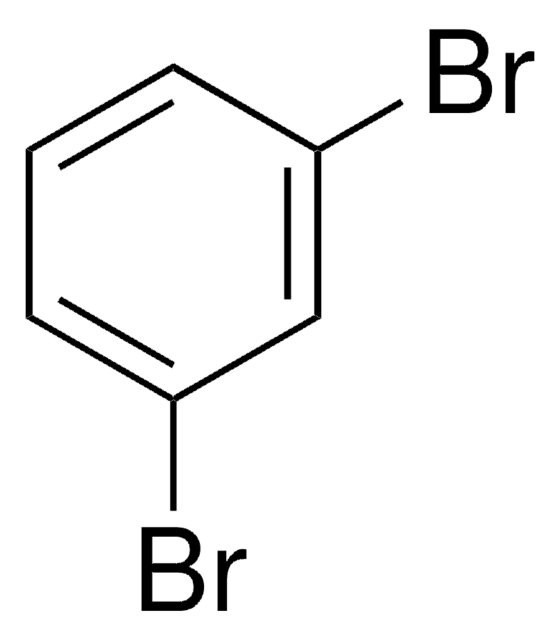
1,2-Dibromobenzene
98%
Synonym(s):
o-Dibromobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4Br2
CAS Number:
Molecular Weight:
235.90
Beilstein:
970241
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
8.2 (vs air)
Assay
98%
form
liquid
refractive index
n20/D 1.611 (lit.)
bp
224 °C (lit.)
mp
4-6 °C (lit.)
density
1.956 g/mL at 25 °C (lit.)
SMILES string
Brc1ccccc1Br
InChI
1S/C6H4Br2/c7-5-3-1-2-4-6(5)8/h1-4H
InChI key
WQONPSCCEXUXTQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,2-Dibromobenzene, also known as o-Dibromobenzene, is an aromatic halogenated hydrocarbon commonly used as a precursor in various organic synthesis reactions based on the intermediate formation of benzynes.
Application
- Selective Covalent Capture of Collagen Triple Helices with Minimal Protecting Group Strategy: This study details the use of Rink Amide MBHA resin for capturing collagen triple helices, highlighting its efficiency in solid-phase synthesis (JDH Le Tracy Yu, 2020).
- Solid phase peptide synthesis: new resin and new protecting group: Introduction of a novel resin, Fmoc-Rink-Amide PEG Octagel, showcasing its application in peptide synthesis (S Ramkisson, 2018).
- Development of a novel, automated, robotic system for rapid, high-throughput, parallel, solid-phase peptide synthesis: This paper discusses the utilization of Rink Amide MBHA resin in developing an automated system for peptide synthesis (K Kiss et al., 2023).
- Synthesis of Peptoids Containing Multiple Nhtrp and Ntrp Residues: A Comparative Study of Resin, Cleavage Conditions and Submonomer Protection: The study compares different resins including Rink Amide MBHA, analyzing their efficacy in peptoid synthesis (A Lone et al., 2020).
It is also used as a key reactant in the synthesis of:
- diborylbenzenes via palladium-catalyzed borylation of bromobenzenes
- o,o′-tri- and -tetrasubstituted biphenyls via aryne cross-coupling reaction
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
197.6 °F - closed cup
Flash Point(C)
92 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Holger F Bettinger et al.
The Journal of organic chemistry, 72(25), 9750-9752 (2007-11-08)
The one-step high-yield synthesis of 1,2-bis(trimethylsilyl)benzene from 1,2-dibromobenzene using tert-butyllithium and trimethylsilyltriflate is reported. A mechanistic investigation shows that 1,2-dilithiobenzene is not an intermediate in this reaction; the coexistence of trimethylsilyltriflate and tert-butyllithium at very low temperatures allows a sequence
G Shakila et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 86, 449-455 (2011-11-25)
The FT-IR and FT-Raman spectra of the compound 1,2-dibromobenzene have been recorded in the region 4000-100cm(-1). The vibrational analysis has been made using HF and DFT (B3LYP and LSDA) level of theory by employing 6-31 +G (d, p) and 6-311
Jadwiga A Szymańska et al.
International journal of occupational medicine and environmental health, 15(4), 375-383 (2003-03-01)
The distribution, excretion and metabolism of 1,4-dibromobenzene (1,4-DBB) and 1,2-dibromobenzene (1,2-DBB), following a single intraperitoneal administration to female Wistar rats, were investigated using radiotracer 3H and GC-MS technique. The maximum level of 3H after 1,4-DBB administration was detected in all
J A Szymańska et al.
Journal of applied toxicology : JAT, 16(1), 35-41 (1996-01-01)
Various doses of dibromobenzene isomers (1,2-dBB, 1,3-dBB, 1,4-dBB) were administered (i.p.) to BALB mice. The levels of reduced glutathione (GSH) and malondialdehyde (MDA) in the liver, and glutamate-pyruvate transaminase (GPT) (EC.2.6.1.2) gamma-glutamyltransferase (gamma-GT) (EC.2.3.2.2) and triglycerides (TG) in the serum
Mónica Carril et al.
Organic letters, 7(22), 4787-4789 (2005-10-21)
[reaction: see text] A new route to oxcarbazepine (Trileptal), the most widely prescribed antiepileptic drug, starting from commercially available 2'-aminoacetophenone and 1,2-dibromobenzene, is reported. The sequentially accomplished key steps are palladium-catalyzed intermolecular alpha-arylation of ketone enolates and intramolecular N-arylation reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service