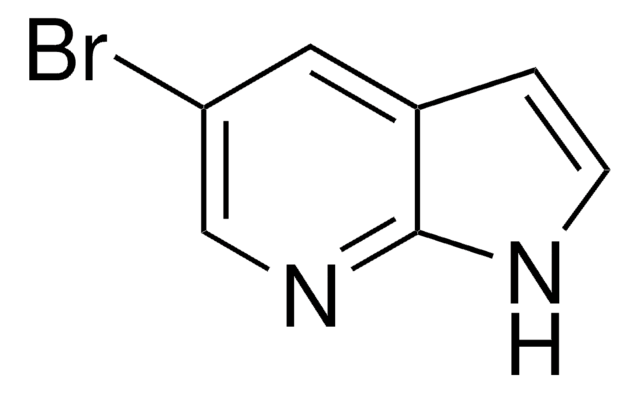
A95502
7-Azaindole
98%
Synonym(s):
1H-Pyrrolo(2,3-b)pyridine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H6N2
CAS Number:
Molecular Weight:
118.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
105-107 °C (lit.)
SMILES string
c1cnc2[nH]ccc2c1
InChI
1S/C7H6N2/c1-2-6-3-5-9-7(6)8-4-1/h1-5H,(H,8,9)
InChI key
MVXVYAKCVDQRLW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
A heterocyclic molecule that can be utilized as a pharmaceutical building block
Starting material in a recent synthesis of azaserotonin.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alastair Donald et al.
Journal of medicinal chemistry, 50(10), 2289-2292 (2007-04-25)
6-phenylpurines were identified as novel, ATP-competitive inhibitors of protein kinase B (PKB/Akt) from a fragment-based screen and were rapidly progressed to potent compounds using iterative protein-ligand crystallography with a PKA-PKB chimeric protein. An elaborated lead compound showed cell growth inhibition
John J Caldwell et al.
Journal of medicinal chemistry, 51(7), 2147-2157 (2008-03-19)
Fragment-based screening identified 7-azaindole as a protein kinase B inhibitor scaffold. Fragment elaboration using iterative crystallography of inhibitor-PKA-PKB chimera complexes efficiently guided improvements in the potency and selectivity of the compounds, resulting in the identification of nanomolar 6-(piperidin-1-yl)purine, 4-(piperidin-1-yl)-7-azaindole, and
Xin Wang et al.
The Journal of organic chemistry, 71(10), 4021-4023 (2006-05-06)
A practical synthesis of a key pharmaceutical intermediate, 2-[(1H-pyrrolo[2,3-b]pyridine-4-yl)methylamino]-5-fluoronicotinic acid (1), is described. To introduce the aminomethyl moiety of 2 via a palladium-catalyzed cyanation/reduction sequence, a regioselective chlorination of 7-azaindole via the N-oxide was developed. A highly selective monodechlorination of
Pei-Wen Wu et al.
Journal of the American Chemical Society, 128(45), 14426-14427 (2006-11-09)
We report the synthesis of 3-(2-aminoethyl)-5-ol-1H-pyrrolo[2,3-b]pyridine (7-azaserotonin), which may potentially serve as an agonist or antagonist of serotonin receptors. In alcohols, the solvent (e.g., ethanol) catalyzed proton-transfer reaction takes place for 7-azaserotonin in the excited state, resulting in dual emission.
Bruno Aleksander Martek et al.
Drug testing and analysis, 11(4), 617-625 (2019-02-08)
The high frequency of the synthetic cannabinoid receptor agonists (SCRAs) emergence renders this group of new psychoactive compounds particularly demanding in terms of detection, identification, and responding. Without the available reference material, one of the specific problems is differentiation and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![1H-pyrrolo[3,2-c]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/291/250/879d6f32-75bf-4a4a-a366-18cbd62dcc37/640/879d6f32-75bf-4a4a-a366-18cbd62dcc37.png)
![1H-Pyrrolo[2,3-c]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/332/393/b23058ac-c477-42b5-9890-dc5c2af5cec7/640/b23058ac-c477-42b5-9890-dc5c2af5cec7.png)
![1H-pyrrolo[3,2-b]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/276/658/7030377c-695d-4006-bbf3-fe99211bb7e7/640/7030377c-695d-4006-bbf3-fe99211bb7e7.png)