803588
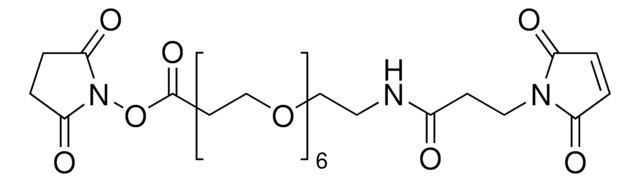
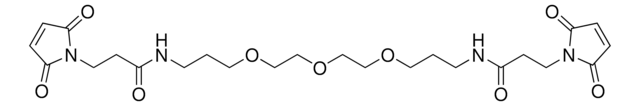
BM(PEG)2 (1,8-bismaleimido-diethyleneglycol)
Synonym(s):
1,2-Bis(2-maleimidoethoxy)ethane, 1,8-Bis(maleimido)-3,6-dioxaoctane
About This Item
Recommended Products
Assay
≥90%
form
powder
mol wt
308.29
reaction suitability
reagent type: cross-linking reagent
storage condition
desiccated
solubility
water: soluble
shipped in
ambient
storage temp.
2-8°C
SMILES string
O=C(C=CC1=O)N1CCOCCOCCN2C(C=CC2=O)=O
InChI
1S/C14H16N2O6/c17-11-1-2-12(18)15(11)5-7-21-9-10-22-8-6-16-13(19)3-4-14(16)20/h1-4H,5-10H2
InChI key
FERLGYOHRKHQJP-UHFFFAOYSA-N
General description
Features and Benefits
- Reactive groups: maleimide (both ends)
- Reactive towards: sulfhydryl groups
- Long, pegylated, sulfhydryl-to-sulfhydryl crosslinkers, composed of maleimide groups and 2-unit polyethylene glycol spacer arm
- PEG spacers provide unique advantages, including enhanced solubility, increased stability, reduced tendency toward aggregation and reduced immunogenicity
- Pure compounds with defined structure and molecular weight, ensuring reproducible protein-modification effects
- Ideal for small molecule or peptide conjugations
Caution
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service