726656
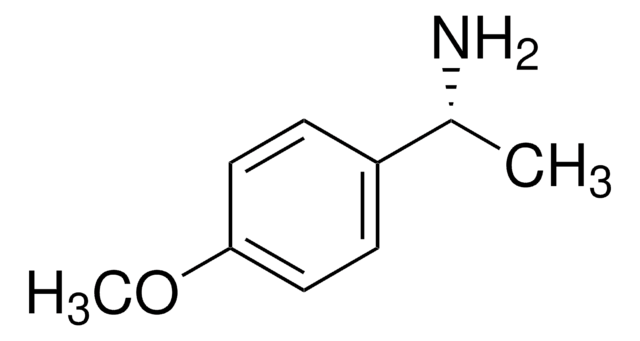
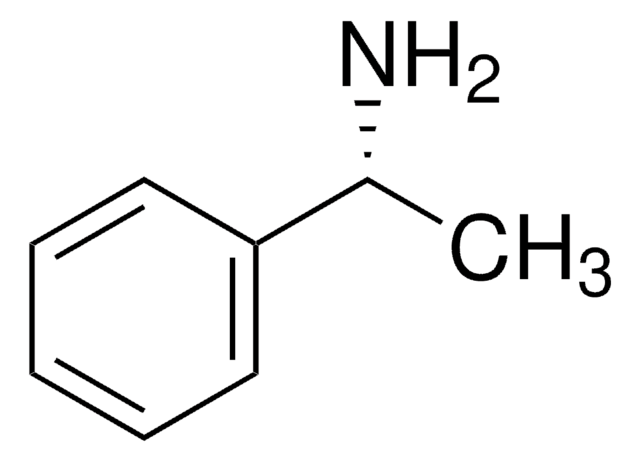
(S)-(−)-4-Methoxy-α-methylbenzylamine
ChiPros®, produced by BASF, 99%
Synonym(s):
(S)-(−)-1-(4-Methoxyphenyl)ethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H13NO
CAS Number:
Molecular Weight:
151.21
Beilstein:
3196456
MDL number:
UNSPSC Code:
12352112
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
produced by BASF
Quality Level
Assay
≥98.5% (GC)
99%
form
liquid
optical purity
enantiomeric excess: ≥98.5%
density
1.024 g/mL at 20 °C (lit.)
functional group
amine
SMILES string
COc1ccc(cc1)[C@H](C)N
InChI
1S/C9H13NO/c1-7(10)8-3-5-9(11-2)6-4-8/h3-7H,10H2,1-2H3/t7-/m0/s1
InChI key
JTDGKQNNPKXKII-ZETCQYMHSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
(S)-(−)-4-Methoxy-α-methylbenzylamine is employed in the synthesis of S(+)-4-(1-phenylethylamino)quinazolines, as human immunoglobuline E inhibitor and haloaryl-β-amino acids. It is also used as a precursor to prepare chiral intermediate in the total synthesis of solanoeclepin A.
Legal Information
ChiPros is a registered trademark of BASF SE
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S (+)-4-(1-phenylethylamino) quinazolines as inhibitors of human immunoglobuline E synthesis: potency is dictated by stereochemistry and atomic point charges at N-1.
Berger M, et al.
Journal of Medicinal Chemistry, 44(18), 3031-3038 (2001)
The asymmetric synthesis of β-haloaryl-β-amino acid derivatives.
Bull S D, et al.
Synlett, 2000(09), 1257-1260 (2000)
Novel Synthesis of the ABC Rings of Solanoeclepin A.
Lin Y T, et al.
Organic Letters, 16(22), 5948-5951 (2014)
Articles
ChiPros Chiral Amines
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service