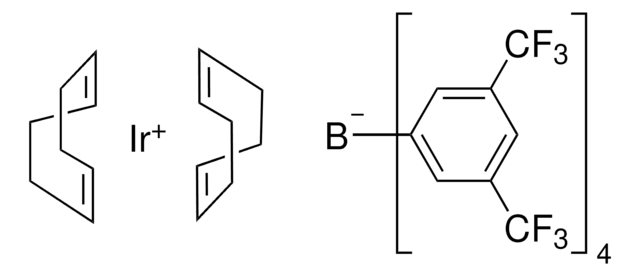
685062
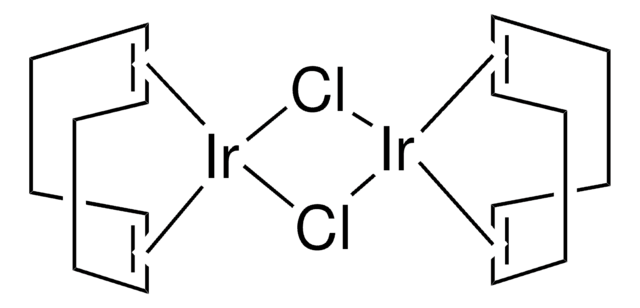
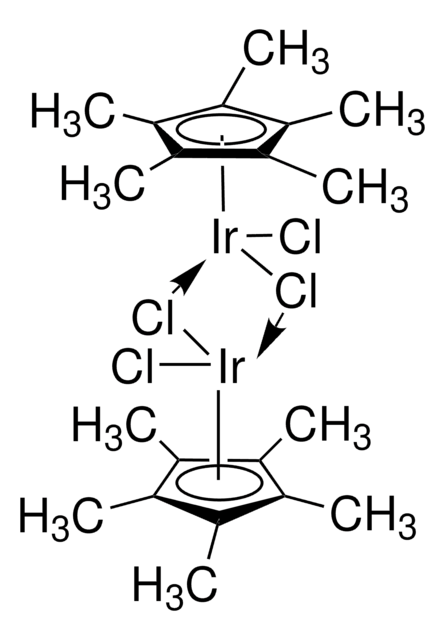
(1,5-Cyclooctadiene)(methoxy)iridium(I) dimer
Synonym(s):
[Ir(OMe)(1,5-cod)]2, Bis(1,5-cyclooctadiene)di-μ-methoxydiiridium(I)
About This Item
Recommended Products
form
crystals
Quality Level
reaction suitability
core: iridium
reagent type: catalyst
reaction type: C-H Activation
mp
154-179 °C (D)
storage temp.
−20°C
SMILES string
C[O+]1[Ir-]2[O+](C)[Ir-]12.C3CC=CCCC=C3.C4CC=CCCC=C4
InChI
1S/2C8H12.2CH3O.2Ir/c2*1-2-4-6-8-7-5-3-1;2*1-2;;/h2*1-2,7-8H,3-6H2;2*1H3;;/q;;2*+1;2*-1/b2*2-1-,8-7-;;;;
InChI key
BGWIAAATAAWGOI-MIXQCLKLSA-N
Application
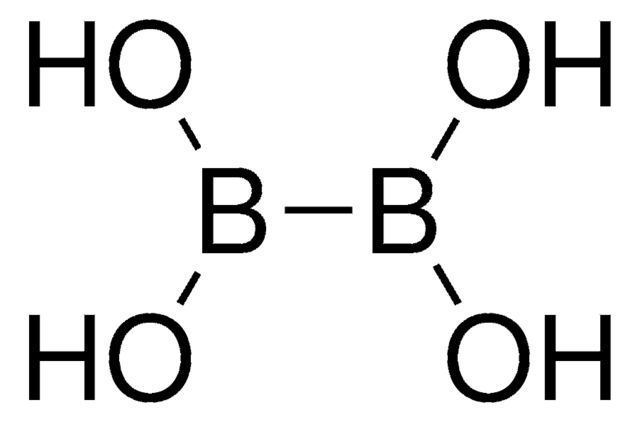
- Preparation of heteroaryl fused indole ring systems as inhibitors of HCV NS5B polymerase
- Borylation/Suzuki-Miyaura coupling
- Metalation-Suzuki cross-coupling procedure for the synthesis of biaryls and heterobiaryls
- Tetraborylation reactions
- Highly regio- and enantioselective asymmetric hydroboration
- Ortho-silylation of aryl ketone, benzaldehyde, and benzyl alcohol derivatives via C-H activation
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Ir(I)-Catalyzed C–H Borylation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)