557927
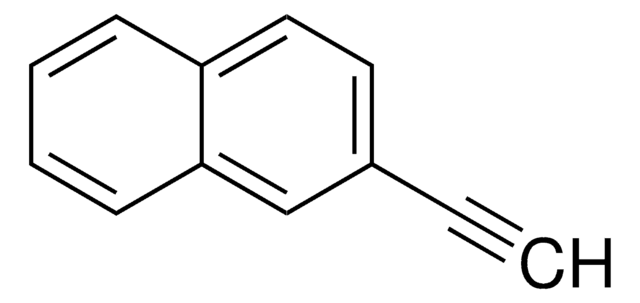
1-Ethynylnaphthalene
97%
Synonym(s):
α-Ethynylnaphthalene, α-Naphthylacetylene, 1-Ethynylnaphthalene, 1-Naphthylacetylene, 1-Naphthylethyne
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C10H7C≡CH
CAS Number:
Molecular Weight:
152.19
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.6500 (lit.)
density
1.070 g/mL at 25 °C (lit.)
SMILES string
C#Cc1cccc2ccccc12
InChI
1S/C12H8/c1-2-10-7-5-8-11-6-3-4-9-12(10)11/h1,3-9H
InChI key
MCZUXEWWARACSP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Ethynylnaphthalene can be prepared from 1-acetonaphthone or 1-naphthyl-2-trimethylsilylacetylene.
Application
1-Ethynylnaphthalene may be used to synthesize bis-6,6′-(ethynyl-1-naphthalene)-2,2′-bipyridine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
136.0 °F - closed cup
Flash Point(C)
57.8 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G J Hammons et al.
Chemical research in toxicology, 2(6), 367-374 (1989-11-01)
Since the N-oxidation of several carcinogenic arylamines has been shown to be catalyzed preferentially by cytochrome P-450IA2 in several species, homologous ethynyl-substituted aromatic hydrocarbons, 2-ethynylnaphthalene, 1-ethynylnaphthalene, and 2-ethynylfluorene, were synthesized and examined as potential mechanism-based inactivators of this monooxygenase. By
Bipyridylacetylenes 1: the synthesis of some bipyridylacetylenes via the palladium-catalyzed coupling of acetylenes with 2, 2'-dibromobipyridyl, and the single crystal X-ray structure of 6, 6'-bisphenylethynyl-2, 2'-bipyridine.
Butler IR and Soucy-Breau C.
Canadian Journal of Chemistry, 69(7), 1117-1123 (1991)
Articles
New Aromatic Terminal Alkynes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service