All Photos(2)
About This Item
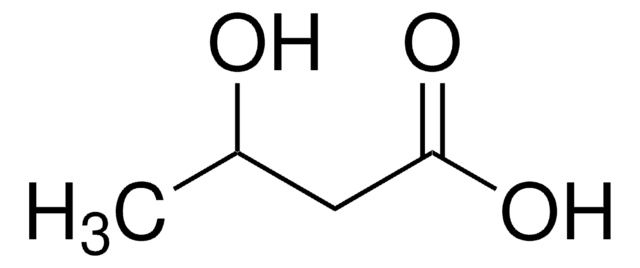
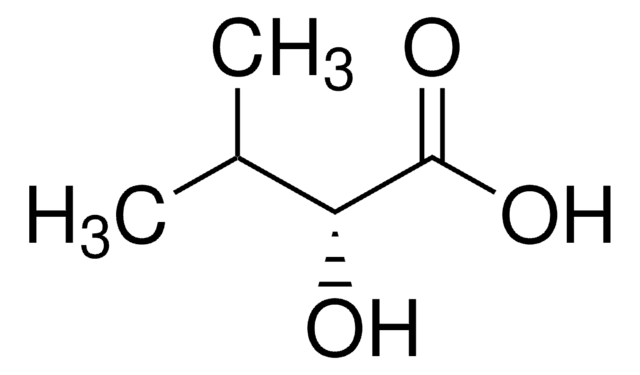
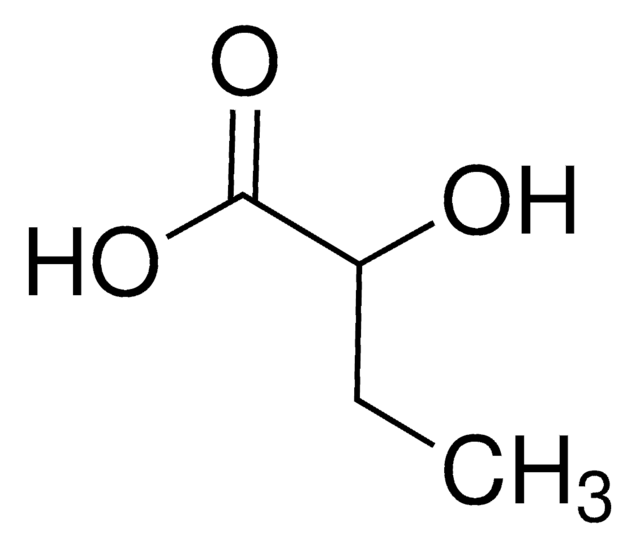
Empirical Formula (Hill Notation):
C4H8O3
CAS Number:
Molecular Weight:
104.10
Beilstein:
1720939
MDL number:
UNSPSC Code:
51113400
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (T)
form
solid
optical purity
enantiomeric ratio: ≥99:1 (GC)
mp
50-54 °C
functional group
carboxylic acid
hydroxyl
storage temp.
2-8°C
SMILES string
CC[C@@H](O)C(O)=O
InChI
1S/C4H8O3/c1-2-3(5)4(6)7/h3,5H,2H2,1H3,(H,6,7)/t3-/m1/s1
InChI key
AFENDNXGAFYKQO-GSVOUGTGSA-N
Other Notes
Chiral building block
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K.J. Hale et al.
Tetrahedron Letters, 36, 6965-6965 (1995)
M N Romanelli et al.
Chirality, 8(8), 579-584 (1996-01-01)
The enantiomers of 3-alpha-tropyl 2-(phenylthio)butyrate (SM32, 1) were prepared by chiral synthesis and tested for analgesic, cognition-enhancing, and ACh-releasing properties. They show enantioselectivity in some of the tests, the eutomer being related in configuration to R-(+)-hyoscyamine.
M N Romanelli et al.
Chirality, 8(3), 225-233 (1996-01-01)
The enantiomers of two alpha-tropanyl esters, SM21 (1) and PG9 (2), derived from (+)-R-hyoscyamine, that act by increasing the central cholinergic tone, were obtained by esterification after resolution of the corresponding racemic acids [(-)-S-1, (-)-R-2 and (+)-S-2] and by stereospecific
Si Jae Park et al.
Applied microbiology and biotechnology, 93(1), 273-283 (2011-08-16)
We have previously reported in vivo biosynthesis of polylactic acid (PLA) and poly(3-hydroxybutyrate-co-lactate) [P(3HB-co-LA)] employing metabolically engineered Escherichia coli strains by the introduction of evolved Clostridium propionicum propionyl-CoA transferase (Pct(Cp)) and Pseudomonas sp. MBEL 6-19 polyhydroxyalkanoate (PHA) synthase 1 (PhaC1(Ps6-19)).
Philip J Saylor et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 18(13), 3677-3685 (2012-05-17)
Androgen deprivation therapy (ADT) for prostate cancer causes an increase in fasting insulin and adverse changes in body composition and serum lipid profile. It is unknown what other metabolic alterations are caused by ADT. To better characterize the metabolic effects
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service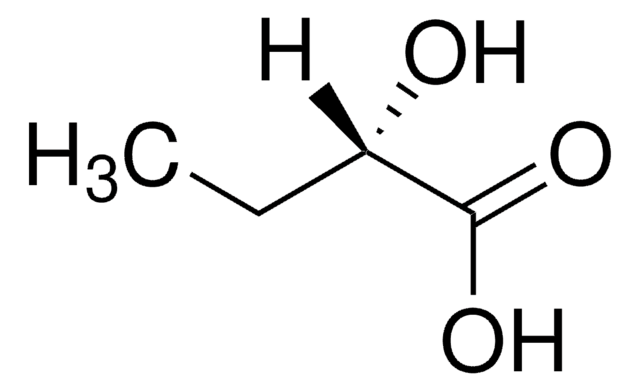

![[(3R)-3-Hydroxybutyryl]-L-carnitine analytical standard](/deepweb/assets/sigmaaldrich/product/structures/658/500/ff9570f8-a346-4077-9983-d0e67400bf47/640/ff9570f8-a346-4077-9983-d0e67400bf47.png)
![[(3R)-3-Hydroxyoctadecanoyl]-L-carnitine analytical standard](/deepweb/assets/sigmaaldrich/product/structures/195/646/9c581614-9449-4107-a05e-7c573a907483/640/9c581614-9449-4107-a05e-7c573a907483.png)