531464
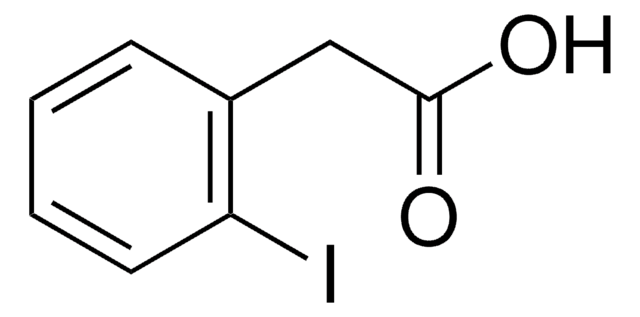
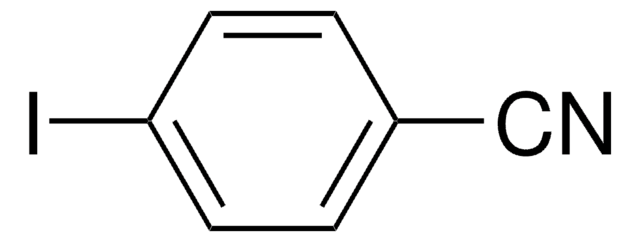
2-Iodophenylacetonitrile
97%
Synonym(s):
2-Iodobenzyl cyanide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
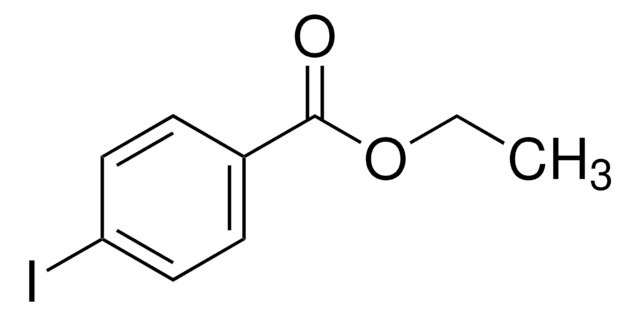
IC6H4CH2CN
CAS Number:
Molecular Weight:
243.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
refractive index
n20/D 1.618 (lit.)
bp
113-120 °C/0.5 mmHg (lit.)
density
1.75 g/mL at 25 °C (lit.)
functional group
iodo
nitrile
SMILES string
Ic1ccccc1CC#N
InChI
1S/C8H6IN/c9-8-4-2-1-3-7(8)5-6-10/h1-4H,5H2
InChI key
FPSGTRJUQLYLHE-UHFFFAOYSA-N
General description
2-Iodophenylacetonitrile is a 2-aryl substituted nitrile. It reacts with lactams to form ring-fused isoquinolinones via palladium-catalyzed carboxamidation in tandem with aldol condensation.
Application
2-Iodophenylacetonitrile may be used in the preparation of:
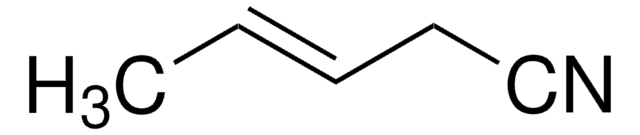
It may also be used in the preparation of the following nitriles:
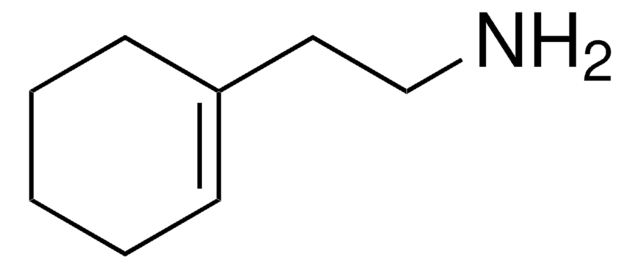
- 2?-aminobiphen-2-ylacetonitrile
- ethyl (2-iodophenyl)iminoacetate hydrochloride
- 3,4-disubstituted 2-naphthalenamines
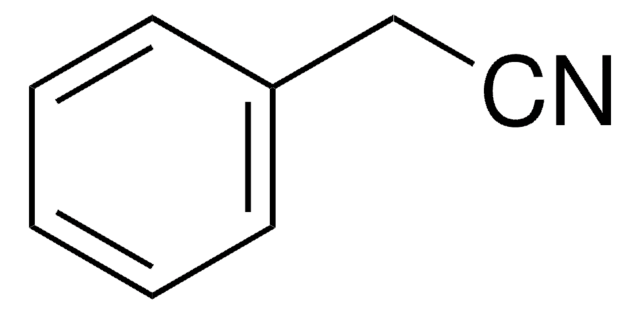
It may also be used in the preparation of the following nitriles:
- 2-(2-iodophenyl)-2-methylpropanenitrile
- 1-(2-iodophenyl)cyclopentanecarbonitrile
- 5-bromo-2-(2-iodophenyl)pentanenitrile
- 2-(2-iodophenyl)-2-propylpentanenitrile
- 1-(2-iodophenyl)cyclohexanecarbonitrile
- 1-(2-Iodophenyl)cyclopropanecarbonitrile
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
David Crich et al.
The Journal of organic chemistry, 71(9), 3452-3463 (2006-04-22)
The [1-cyano-2-(2-iodophenyl)]ethylidene group is introduced as an acetal-protecting group for carbohydrate thioglycoside donors. The group is easily introduced under mild conditions, over short reaction times, and in the presence of a wide variety of other protecting groups by the reaction
Hirokazu Tsukamoto et al.
The Journal of organic chemistry, 81(5), 1733-1745 (2015-11-26)
1,2-Bis(diphenylphosphino)ethane (dppe)-ligated palladium(II) complexes catalyze the annulation of internal alkynes with 2-(cyanomethyl)phenylboronates to provide 3,4-disubstituted-2-naphthalenamines in good yields. The annulation reaction proceeds under mild and neutral conditions and requires methanol as an essential solvent. In addition to symmetrical alkynes, unsymmetrical
Studies in Acyl C? H Activation via Aryl and Alkyl to Acyl ?Through Space? Migration of Palladium.
Kesharwani T, et al.
Organic Letters, 11(12), 2591-2593 (2009)
Palladium-catalyzed carboxamidation reaction and aldol condensation reaction cascade: A facile approach to ring-fused isoquinolinones.
Chouhan G and Alper H.
Organic Letters, 10(21), 4987-4990 (2008)
Palladium-catalyzed borylation of ortho-substituted phenyl halides and application to the one-pot synthesis of 2,2'-disubstituted biphenyls.
Baudoin O, et al.
The Journal of Organic Chemistry, 65(26), 9268-9271 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service