All Photos(1)
About This Item
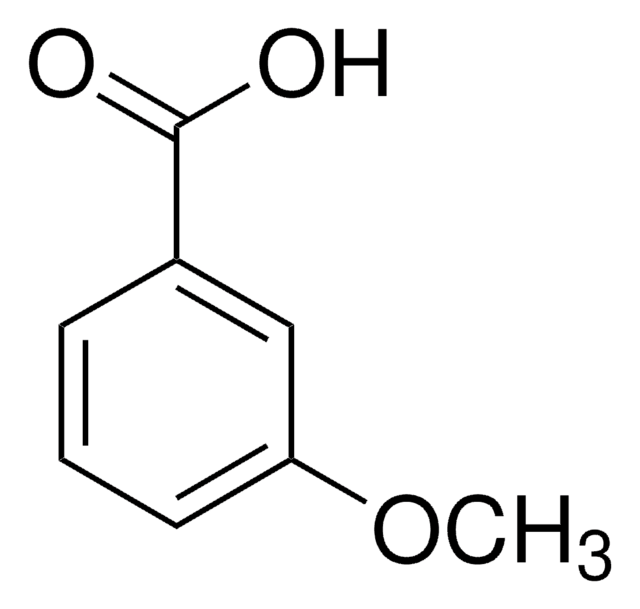
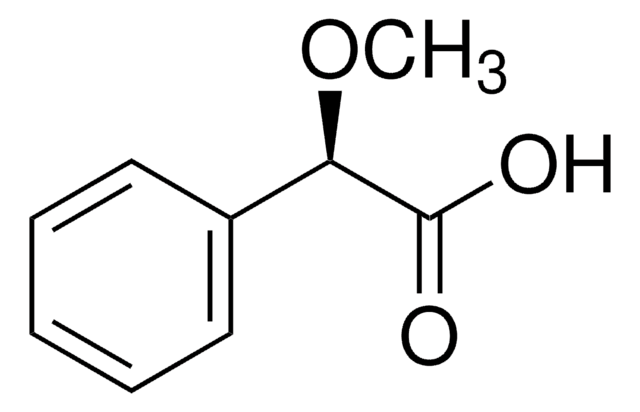
Linear Formula:
CH3OC6H3(CH3)CO2H
CAS Number:
Molecular Weight:
166.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
177-181 °C (lit.)
functional group
carboxylic acid
SMILES string
COc1ccc(C(O)=O)c(C)c1
InChI
1S/C9H10O3/c1-6-5-7(12-2)3-4-8(6)9(10)11/h3-5H,1-2H3,(H,10,11)
InChI key
MSVRGYOYISBGTH-UHFFFAOYSA-N
General description
4-Methoxy-2-methylbenzoic acid (2-methylanisic acid) is a trisubstituted aromatic compound. It is a positional isomeric form of 2-methoxy-4-methylbenzoic acid. 4-Methoxy-2-methylbenzoic acid readily forms complexes with Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II).
Application
4-Methoxy-2-methylbenzoic acid may be used in the synthesis of 4-methoxy-2-methylbenzoates of Y(III) and lanthanides (III) (La to Lu, excluding Pm). It may also be used to synthesize 4-methoxy-2-methylbenzoic acid hydrazide and 5-bromo-4-methoxy-2-methylbenzoic acid.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Spectral and thermal investigation of rare earth element 4-methoxy-2-methylbenzoates.
Brzyska W and Ozga W.
Journal of Thermal Analysis and Calorimetry, 70(2), 467-474 (2002)
A Omodei-Salé et al.
Journal of medicinal chemistry, 26(8), 1187-1192 (1983-08-01)
A series of 3,5-diaryl-s-triazoles were synthesized and evaluated as postimplantation contragestational agents. The introduction of various substituents (e.g., an o-alkyl chain on one phenyl and a m-alkoxy group on the other) was found to increase the potency. Several compounds with
Peng Hu et al.
Organic letters, 11(11), 2341-2344 (2009-05-13)
A versatile palladium catalyst system was developed to effect the decarboxylative Heck coupling of a variety of arenecarboxylic acids with a wide range of olefins. The key to obtaining the efficient catalyst system is the use of 1-adamatanecarboxylic acid as
(1 S)-1, 5-Anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-d-glucitol (TS-071) is a potent, selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes treatment.
Kakinuma H, et al.
Journal of Medicinal Chemistry, 58(3), 3247-3261 (2010)
Thermal, spectral and magnetic investigations of Mn (II), Co (II), Ni (II), Cu (II), Zn (II) and cd (ii) complexes with 4-methoxy-2-methylbenzoic acid.
Meganathan C, et al.
Polish Journal of Chemistry, 76(12), 1689-1692 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service