All Photos(1)
About This Item
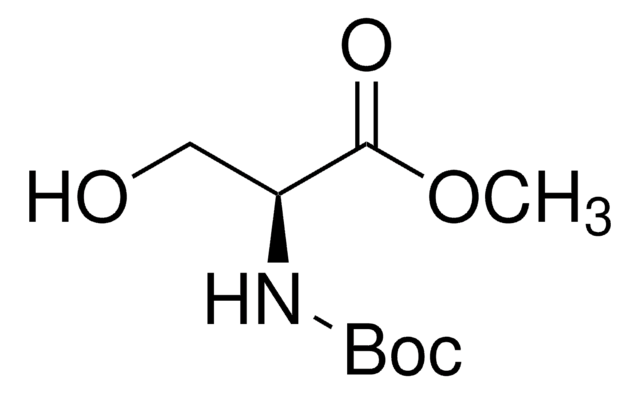
Linear Formula:
HOCH2CH(NHCO2CH2C6H5)CO2CH3
CAS Number:
Molecular Weight:
253.25
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
reaction suitability
reaction type: solution phase peptide synthesis
bp
170 °C/0.01 mmHg (lit.)
mp
41-43 °C (lit.)
application(s)
peptide synthesis
SMILES string
COC(=O)[C@H](CO)NC(=O)OCc1ccccc1
InChI
1S/C12H15NO5/c1-17-11(15)10(7-14)13-12(16)18-8-9-5-3-2-4-6-9/h2-6,10,14H,7-8H2,1H3,(H,13,16)/t10-/m0/s1
InChI key
CINAUOAOVQPWIB-JTQLQIEISA-N
Application
This protected serine has been employed as a starting material for the Cbz analog of Garner′s aldehyde, 2,3-diaminopropanol, and optically active derivatives of 2-amino-1,3-propanediol.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Demirci, F. et al.
Synthesis, 189-189 (1996)
Kang, M. et al.
The Journal of Organic Chemistry, 61, 5528-5528 (1996)
Monache, G.D. et al.
Synthesis, 1155-1155 (1995)
James A. Marshall et al.
The Journal of organic chemistry, 61(2), 581-586 (1996-01-26)
The aminoheptose destomic acid (3.5) and the aminooctose lincosamine (6.8) were synthesized in protected form by parallel sequences starting from the oxazolidine derivatives 2.4 and 5.1 of N-CBz serinal and N-BOC threoninal. The parallel sequences feature BF(3)-promoted addition of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service