454273
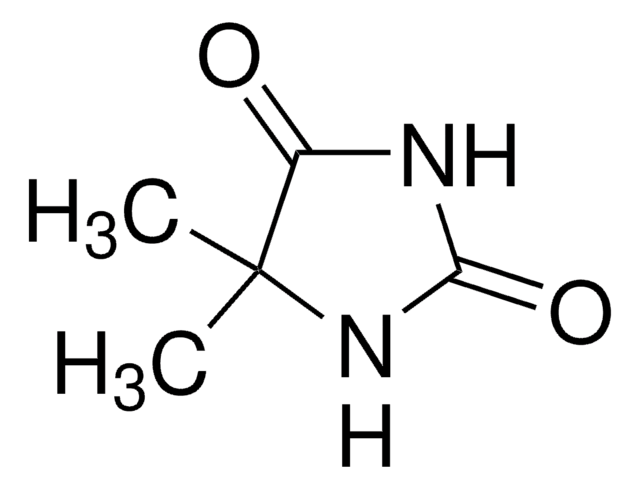
5-Ethyl-5-methylhydantoin
97%
Synonym(s):
5-Ethyl-5-methyl-2,4-imidazolidinedione, NSC 1020
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10N2O2
CAS Number:
Molecular Weight:
142.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
144-150 °C (lit.)
SMILES string
CCC1(C)NC(=O)NC1=O
InChI
1S/C6H10N2O2/c1-3-6(2)4(9)7-5(10)8-6/h3H2,1-2H3,(H2,7,8,9,10)
InChI key
VSJRBQDMBFFHMC-UHFFFAOYSA-N
Application
Reactant for synthesis of nociceptin/orphanin FQ analogues
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Preferential crystallisation and comparative crystal growth study between pure enantiomer and racemic mixture of a chiral molecule: 5-ethyl-5-methylhydantoin.
Beilles S, et al.
Chemical Engineering Science, 56(7), 2281-2294 (2001)
Palash K Sarker et al.
International journal of molecular sciences, 13(1), 1006-1017 (2012-02-09)
Aqueous solutions of isovaline and its precursor molecule, 5-ethyl-5-methylhydantoin, were irradiated with ultraviolet and γ-ray photons, to evaluate their structural stability against space radiation. The degree of photolysis was measured and irradiation products were identified using chiral, reversed-phase and ion-exchange
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service