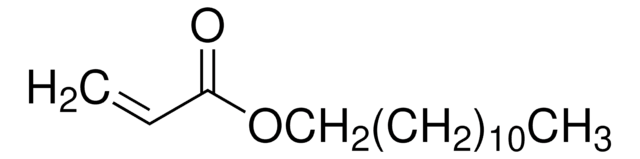408271
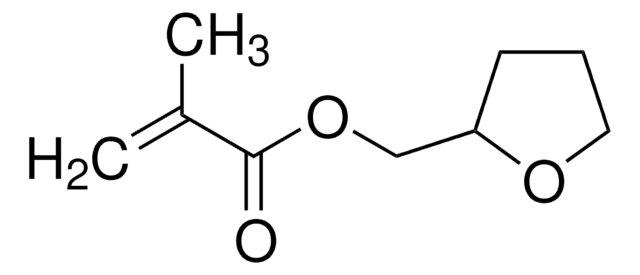
Tetrahydrofurfuryl acrylate
contains 500 ppm hydroquinone as inhibitor, 500 ppm monomethyl ether hydroquinone as inhibitor
Synonym(s):
THFA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H12O3
CAS Number:
Molecular Weight:
156.18
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
liquid
Quality Level
contains
500 ppm hydroquinone as inhibitor
500 ppm monomethyl ether hydroquinone as inhibitor
refractive index
n20/D 1.46 (lit.)
bp
87 °C/9 mmHg (lit.)
density
1.064 g/mL at 25 °C (lit.)
SMILES string
C=CC(=O)OCC1CCCO1
InChI
1S/C8H12O3/c1-2-8(9)11-6-7-4-3-5-10-7/h2,7H,1,3-6H2
InChI key
YNXCGLKMOXLBOD-UHFFFAOYSA-N
Related Categories
Application
Tetrahydrofurfuryl acrylate may be used as an acrylic matrix for silver nanoparticles/polymer nanocomposites . It can form copolymers with butadiene. Prior to transfer printing, cellulosic and proteinic fibers are grafted with THFA.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A new and convenient route to polyacrylate/silver nanocomposites by light-induced cross-linking polymerization
Balan L, et al.
Progress in Organic Coatings, 62(3), 351-357 (2008)
Fan Xie et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 22(5), 455-460 (2021-01-17)
A chiral adduct formed between a chiral carboxylic acid, tetrahydro-2-furoic acid (THFA), and a chiral ester, propylene oxide (PO), was investigated using rotational spectroscopy and DFT calculations. Isolated THFA exists dominantly as three different conformers: I, II, and III in
David A Korasick et al.
Biophysical journal, 114(12), 2833-2843 (2018-06-21)
Homooligomerization of proline utilization A (PutA) bifunctional flavoenzymes is intimately tied to catalytic function and substrate channeling. PutA from Bradyrhizobium japonicum (BjPutA) is unique among PutAs in that it forms a tetramer in solution. Curiously, a dimeric BjPutA hot spot
Transfer printing of cellulosic and proteinic fabrics.
El-Molla MM, et al.
Advances in Polymer Technology, 20(4), 296-304 (2001)
Some new butadiene copolymers.
Marvel CS, et al.
Journal of Polymer Science, 8(6), 599-605 (1952)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service