All Photos(2)
About This Item
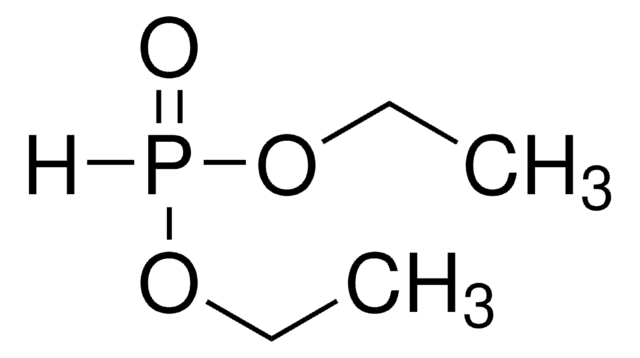
Linear Formula:
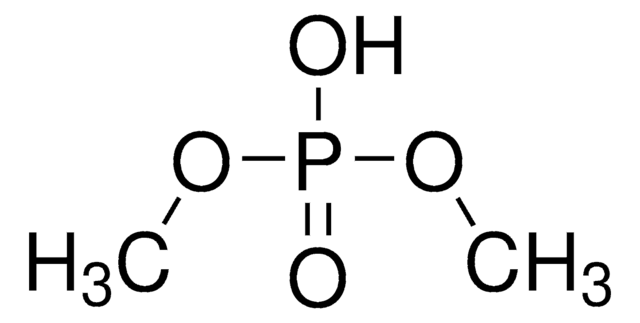
(C2H5O)2P(O)H
CAS Number:
Molecular Weight:
138.10
Beilstein:
605759
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
94%
form
liquid
refractive index
n20/D 1.407 (lit.)
bp
50-51 °C/2 mmHg (lit.)
density
1.072 g/mL at 25 °C (lit.)
SMILES string
[H]P(=O)(OCC)OCC
InChI
1S/C4H11O3P/c1-3-6-8(5)7-4-2/h8H,3-4H2,1-2H3
InChI key
MJUJXFBTEFXVKU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Diethyl phosphite is reported to be chemical warfare agent (CWA) simulant, simulant for nerve agents sarin, soman, tabun, and VX.
Application
Diethyl phosphite may be used to prepare the nickel chloride-diethyl phosphite system, efficient catalyst for the cross-coupling reaction between various functionalized arylzinc halides and aryl bromides, triflates and activated chlorides. It may be used:
- in the stereoselective synthesis of β-fluorinated alkylphosphonates
- in the synthesis of homoallylic bromide
- in the synthesis of series of carbazole-based α-aminophosphonates
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Sens. 1B
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wenke Qi et al.
The Journal of organic chemistry, 78(12), 5918-5924 (2013-05-22)
Cyclopropyl Grignard reagents react with carbonyl compounds in the presence of diethyl phosphite to give homoallylic bromides. The reaction is effectively carried out under mild conditions in a one-pot fashion with moderate to good yields.
Adam M Graichen et al.
Journal of the American Society for Mass Spectrometry, 24(6), 917-925 (2013-03-28)
The gas-phase reactions of a series of coordinatively unsaturated [Ni(L)n](y+) complexes, where L is a nitrogen-containing ligand, with chemical warfare agent (CWA) simulants in a miniature rectilinear ion trap mass spectrometer were investigated as part of a new approach to
Anil Kumar Mungara et al.
Chemical & pharmaceutical bulletin, 60(12), 1531-1537 (2012-09-19)
A novel series of carbazole-based α-aminophosphonates were synthesized by three component coupling of 6-bromo-9-ethyl-9H-carbazole-3-carbaldehyde, amine and diethyl phosphite using polyethylene glycol (PEG-400) as a green reaction media. The antiproliferative activity of these molecules was evaluated against three cancer cell lines.
Andrei Gavryushin et al.
Organic letters, 7(22), 4871-4874 (2005-10-21)
[reaction: see text] The combination of diethyl phosphite and DMAP as ligands for nickel in an 8:1 THF-N-ethylpyrrolidinone (NEP) mixture allows a very efficient cross-coupling reaction to be performed between various functionalized arylzinc halides and aryl bromides, triflates and activated
Chengwei Zhang et al.
Journal of the American Chemical Society, 135(38), 14082-14085 (2013-09-13)
We report herein a mild and catalytic phosphonofluorination of unactivated alkenes. With catalysis by AgNO3, the condensation of various unactivated alkenes with diethyl phosphite and Selectfluor reagent in CH2Cl2/H2O/HOAc at 40 °C led to the efficient synthesis of β-fluorinated alkylphosphonates
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service