359785
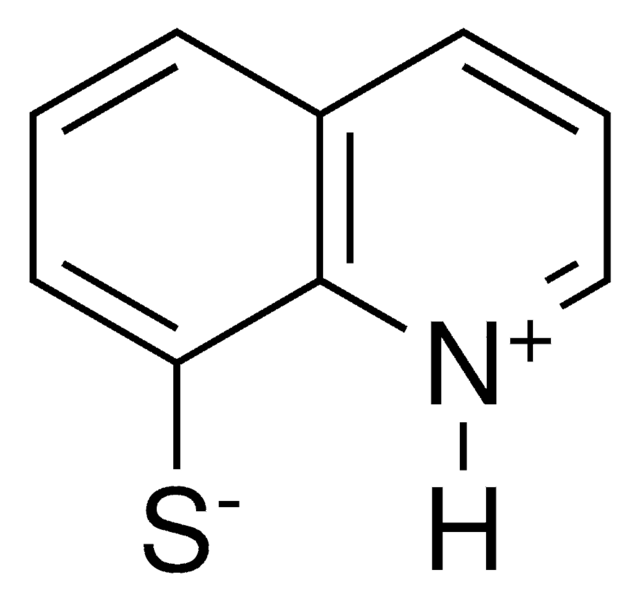
8-Quinolinethiol hydrochloride
97%
Synonym(s):
8-Mercaptoquinoline hydrochloride, Thiooxine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H7NS · HCl
CAS Number:
Molecular Weight:
197.68
Beilstein:
3694939
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
165 °C (dec.) (lit.)
solubility
ethanol: soluble 50 mg/mL, clear, dark orange-brown
SMILES string
Cl[H].Sc1cccc2cccnc12
InChI
1S/C9H7NS.ClH/c11-8-5-1-3-7-4-2-6-10-9(7)8;/h1-6,11H;1H
InChI key
RWBSBQAUAJSGHY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
8-Quinolinethiol hydrochloride may be used in the synthesis of N2S2-donor tetradentate ligands.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
N Ashizawa et al.
Biological & pharmaceutical bulletin, 17(2), 207-211 (1994-02-01)
The effects of various metal chelators on endothelin (ET)-converting enzyme (ECE) activity were examined in vitro. Three chelators, 2,3-dimercapto-1-propanol (DMP), toluene-3,4-dithiol (TDT) and 8-mercaptoquinoline (8-MQ), were found to dose-dependently inhibit ECE activity, but this inhibition was much weaker compared with
Synthesis and coordination chemistry of tetradentate ligands containing two bidentate thioquinoline units: mononuclear complexes with Cu (I) and Cu (II), and a coordination polymer with Cu (I).
Tavacoli S, et al.
Polyhedron, 22(4), 507-514 (2003)
N Ashizawa et al.
Biological & pharmaceutical bulletin, 17(2), 212-216 (1994-02-01)
The effects of metal chelators on endothelin (ET)-converting enzyme (ECE) activity in vivo were examined. Three compounds, (2,3-dimercapto-1-propanol (DMP), toluene-3,4-dithiol (TDT) and 8-mercaptoquinoline (8-MQ)), which inhibited ECE in in vitro studies, exhibited inhibitory activity towards big ET-1-induced sudden death in
K Borg-Neczak et al.
Pharmacology & toxicology, 74(3), 185-192 (1994-03-01)
Oral administration of Ni2+ together with 8-hydroxyquinoline (8-OH-quinoline), 8-mercaptoquinoline (8-SH-quinoline) or 5-chloro-7-iodo-8-hydroxyquinoline (clioquinol) resulted in increased tissue levels of the metal in several tissues of mice in comparison with animals given the Ni2+ alone. Ni2+ forms lipophilic complexes with these
W D Figg et al.
Journal of chromatography, 652(2), 187-194 (1994-02-11)
This paper describes a reversed-phase, high-performance liquid chromatographic (HPLC) method for the isolation, detection, and quantification of TNP-470 (I) and one of its active metabolites, AGM-1883 (II), from plasma. These compounds are initially extracted from plasma with an organic solvent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service