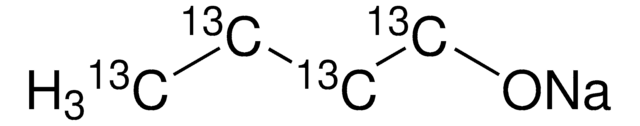359270
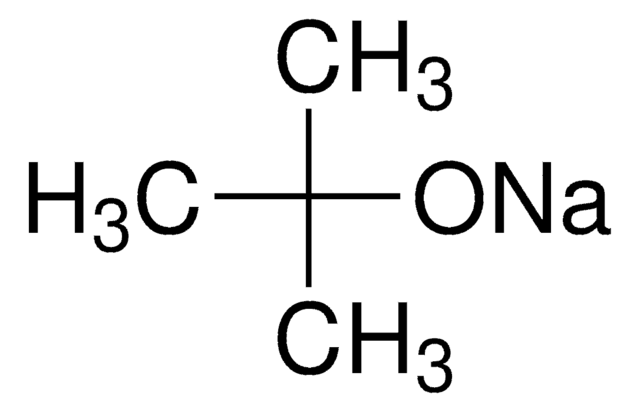
Sodium tert-butoxide
97%
Synonym(s):
Sodium 2-methylpropan-2-olate, Sodium t-butoxide, Sodium tert-butanolate, Sodium tert-butylate
Sign Into View Organizational & Contract Pricing
All Photos(6)
About This Item
Linear Formula:
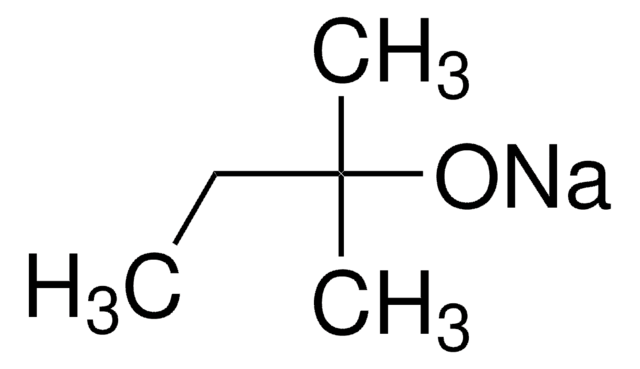
NaOC(CH3)3
CAS Number:
Molecular Weight:
96.10
Beilstein:
3654215
EC Number:
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
SMILES string
[Na+].CC(C)(C)[O-]
InChI
1S/C4H9O.Na/c1-4(2,3)5;/h1-3H3;/q-1;+1
InChI key
MFRIHAYPQRLWNB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Sodium tert-butoxide may be used as a safe and effective alternative to sodium hydride for the coupling of hydroxyethyl adenine with diethyl p-toluenesulfonyloxymethanephosphonate to form 9-[2-(diethylphosphonomethoxy)ethyl]adenine (diethyl-PMEA), which is a key intermediate to prepare adefovir dipivoxil.
It can also be used:
It can also be used:
- To facilitate the coupling of aryl halides with benzene derivatives in the presence of 10-phenanthroline derivative.
- To activate first-row transition-metal pre-catalysts.
- As a base for the amination of aryl chlorides in the presence of a novel palladacyclic precatalyst.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Sol. 1 - Self-heat. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flash Point(F)
57.2 °F
Flash Point(C)
14 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Process optimization in the synthesis of 9-[2-(diethylphosphonomethoxy) ethyl] adenine: replacement of sodium hydride with sodium tert-butoxide as the base for oxygen alkylation.
Yu RH, et al.
Organic Process Research & Development, 3(1), 53-55 (1999)
Activation and discovery of earth-abundant metal catalysts using sodium tert-butoxide.
Docherty JH, et al.
Nature Chemistry, 9(6), 595-595 (2017)
tert-Butoxide-mediated arylation of benzene with aryl halides in the presence of a catalytic 1, 10-phenanthroline derivative.
Shirakawa E, et al.
Journal of the American Chemical Society, 132(44), 15537-15539 (2010)
An air and thermally stable one-component catalyst for the amination of aryl chlorides.
Zim D and Buchwald SL.
Organic Letters, 5(14), 2413-2415 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service