All Photos(1)
About This Item
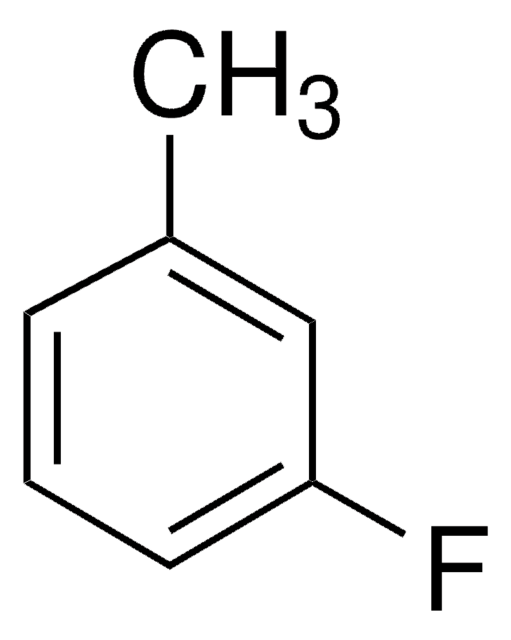
Linear Formula:
CH3C6H3F2
CAS Number:
Molecular Weight:
128.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.453 (lit.)
bp
112 °C/740 mmHg (lit.)
density
1.129 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
Cc1c(F)cccc1F
InChI
1S/C7H6F2/c1-5-6(8)3-2-4-7(5)9/h2-4H,1H3
InChI key
MZLSNIREOQCDED-UHFFFAOYSA-N
General description
2,6-Difluorotoluene reacts with chlorine to give 2,6-difluorobenzyl chloride, which gets converted to 2,6-difluorobenzaldehyde through Sommelet′s reaction. Six-fold potential for internal methyl rotation in first singlet excited state and cation ground state of 2,6-difluorotoluene has been determined.
Application
2,6-Difluorotoluene was used to generate jet-cooled 2,6-difluorobenzyl radical and to investigate its vibronically resolved emission spectra.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
50.0 °F - closed cup
Flash Point(C)
10 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Observation of vibronic emission spectrum of the jet-cooled 2, 6-difluorobenzyl radical.
Lee SK and Baek DY.
The Journal of Physical Chemistry A, 104(22), 5219-5221 (2000)
Methyl Group Internal Rotation in 2, 6-Difluorotoluene (S1) and 2, 6-Difluorotoluene+ (D0).
Walker RA, et al.
The Journal of Physical Chemistry, 99(33), 12422-12433 (1995)
Preparation of 2, 6-difluoro-n-alkylbenzenes from 1, 3-difluorobenzene Transformation of 2, 6-difluorotoluene to the corresponding benzaldehyde via benzyl chloride.
Malykhin EV and Shteingarts VD.
Journal of Fluorine Chemistry, 9(1), 19-20 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[Bis(trifluoroacetoxy)iodo]benzene 97%](/deepweb/assets/sigmaaldrich/product/structures/238/293/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4/640/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4.png)