329606
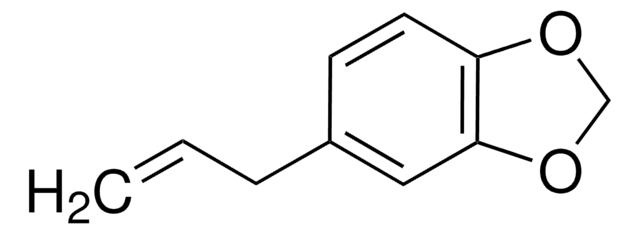
Isosafrol
mixture of cis and trans
Synonym(s):
1,2-Methylenedioxy-4-propenylbenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H10O2
CAS Number:
Molecular Weight:
162.19
Beilstein:
82640
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
refractive index
n20/D 1.573 (lit.)
bp
77-86 °C/3.5 mmHg (lit.)
density
1.12 g/mL at 25 °C (lit.)
SMILES string
C\C=C\c1ccc2OCOc2c1
InChI
1S/C10H10O2/c1-2-3-8-4-5-9-10(6-8)12-7-11-9/h2-6H,7H2,1H3/b3-2+
InChI key
VHVOLFRBFDOUSH-NSCUHMNNSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Isosafrol is also referred as 1,2-methylenedioxy-4(1-propenyl)benzene. It is an important intermediate for the synthesis of drugs like L-DOPA. Peracid oxidation of isosafrole yields isomers of 2,4-dimethyl-3,5-bis(3,4-methylenedioxyphenyl)tetrahydrofuran. Encapsulated vanadyl compounds in Y-zeolite pores catalyzed isosafrol oxidation under microwave irradiation was reported.
Application
Isosafrol was used in preparation of 1-(3,4-methylenedioxyphenyl)-2-propanone (MDP-2-P or PMK).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D Taras-Valéro et al.
Environmental and molecular mutagenesis, 32(4), 314-324 (1999-01-09)
Mice of the XVIInc/Z and DBA/2N strains, which are responsive and nonresponsive, respectively, to the aryl hydrocarbon (Ah) receptor, were treated with the hepatocarcinogen 5,9-dimethyldibenzo[c,g]carbazole and their livers were examined by nuclease P1-enhanced 32P-postlabeling for the levels of DNA adducts
M H Slawson et al.
Toxicology letters, 85(1), 29-34 (1996-04-01)
Within the selective induction of phase II enzymes following treatment with dipyridyls or N-heterocyclic analogs of phenanthrene, strong correlations (r > or = 0.70) are observed between the increase of microsomal epoxide hydrolase (mEH) activity and UDP-glucuronosyltransferase (UGT) activities towards
M A Sarkar et al.
Drug metabolism and disposition: the biological fate of chemicals, 22(6), 827-834 (1994-11-01)
Polycyclic aromatic hydrocarbons present in cigarette smoke induce cytochromes P4501A1 and P4501A2. These isozymes are of toxicological importance because they convert several environmental pollutants to reactive intermediates that form covalent adducts with cellular DNA resulting in mutations and/or malignant transformations.
M Cox et al.
Forensic science international, 179(1), 44-53 (2008-05-30)
In this work, isomers of 2,4-dimethyl-3,5-bis(3,4-methylenedioxyphenyl)tetrahydrofuran (11) are presented as chemical markers formed during the peracid oxidation of isosafrole. The stereochemical configurations of the major and next most abundant diastereoisomer are presented. Also described is the detection of isomers of
E I Shvartz et al.
Voprosy meditsinskoi khimii, 44(2), 167-171 (1998-06-23)
The transcription level of CYP2B1/2 gene in the liver of Sprague-Dawley (SD), Brattleboro (BL) and Wistar (W) rats treated with isosafrol (IS), Arochlor 1254 (AC), phenobarbital (PB) and triphenildioxane (TPD) was studied. The quantity of CYP2B1/2 mRNA was assessed by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service