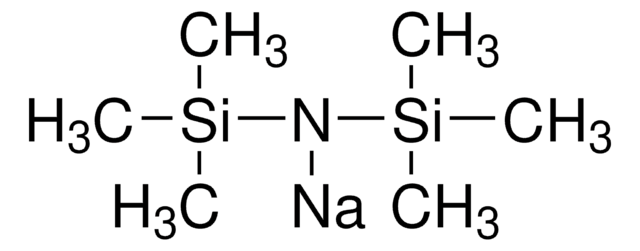329576
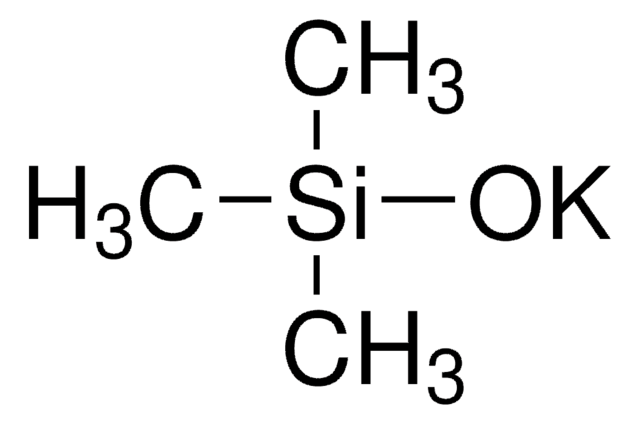
Sodium trimethylsilanolate
95%
Synonym(s):
Trimethylsilanol sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)3SiONa
CAS Number:
Molecular Weight:
112.18
Beilstein:
3912148
EC Number:
MDL number:
UNSPSC Code:
12352000
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
powder
mp
230 °C ((lit.))
230 °C (dec.) (lit.)
SMILES string
[[Na+].C[Si](C)(C)[O-]]
[Na+].C[Si](C)(C)[O-]
InChI
[1S/C3H9OSi.Na/c1-5(2,3)4;/h1-3H3;/q-1;+1]
1S/C3H9OSi.Na/c1-5(2,3)4;/h1-3H3;/q-1;+1
InChI key
[HSNUIYJWTSJUMS-UHFFFAOYSA-N]
HSNUIYJWTSJUMS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
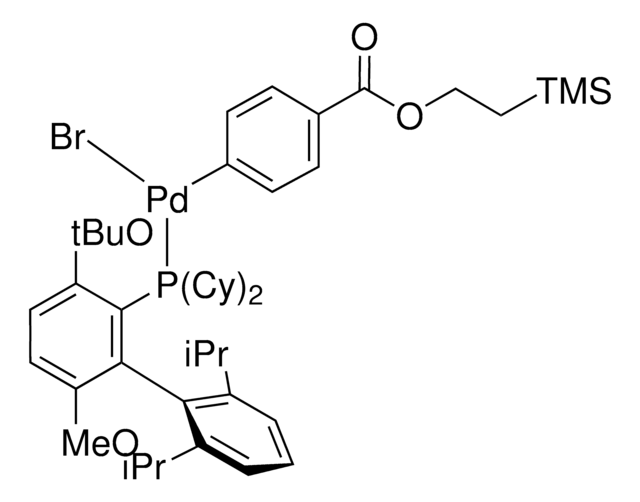
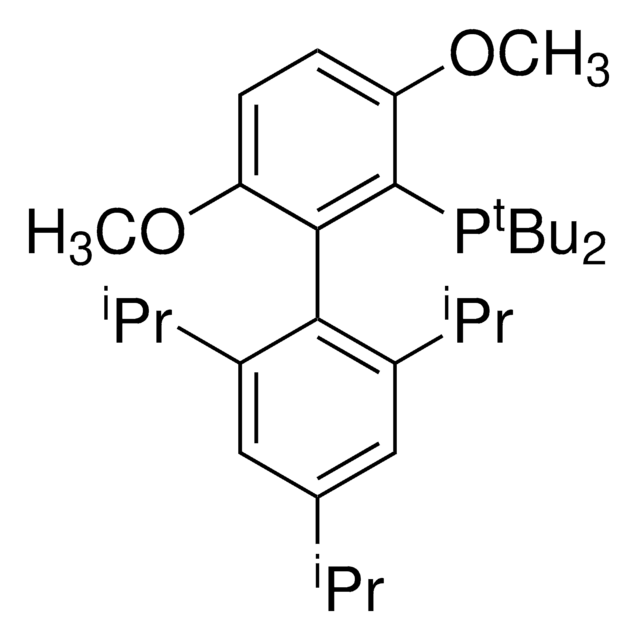
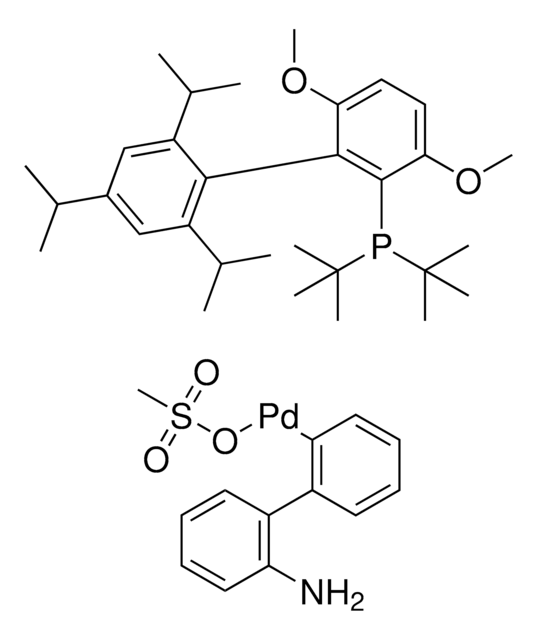
Sodium trimethylsilanolate is a versatile and very powerful reagent for the conversion of esters to carboxylic acids and the hydrolysis of nitriles to primary amides. It can be used as a starting material for the synthesis of metal silanolates via the salt metathesis and as a catalyst for the silylation of silanols with hydrosilanes.
Application
Sodium trimethylsilanolate is used as a catalyst in the:
- Synthesis of the rhodium silonate complex.
- Silylation of methylphenylsilane with tert-butyldimethylsilanol to synthesize trisiloxanes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Scope and limitations of sodium and potassium trimethylsilanolate as reagents for conversion of esters to carboxylic acids
Lovric M, et al.
Croatica Chemica Acta. Arhiv Za Kemiju, 80, 109-115 (2007)
D M Hui et al.
Clinica chimica acta; international journal of clinical chemistry, 302(1-2), 171-188 (2000-11-14)
We developed a new assay method for fluoride anion (F(-)) a specific metabolite of sarin. Trimethyifluorosilane (TMFS) was derivatized from F(-) with trimethylsilanol, and TMFS was detected with a GC-flame ionization detector (FID) and capillary column system. The linear range
A Isquith et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 26(3), 263-266 (1988-03-01)
Six organosilicon compounds which had been found to have clastogenic activity in an in vitro battery of genotoxicity assays were evaluated in rat bone marrow cytogenetic assays for assessing clastogenicity in an in vivo system. None of the six compounds
Igor S Ignatyev et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 60(5), 1169-1178 (2004-04-16)
The assignment of the SiOH group vibrations of trimethylsilanol, which is still controversial, is proposed. This assignment is based on theoretical B3LYP force field scaled using the constants of the (CH3)3Si group optimized to fit experimental vibrational frequencies of (CH3)3SiF
Fabricio A Hansel et al.
Rapid communications in mass spectrometry : RCM, 25(13), 1893-1898 (2011-06-04)
A methodology is presented for the determination of dihydroxy fatty acids preserved in the 'bound' phase of organic residues preserved in archaeological potsherds. The method comprises saponification, esterification, silica gel column chromatographic fractionation, and analysis by gas chromatography/mass spectrometry. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service