329517
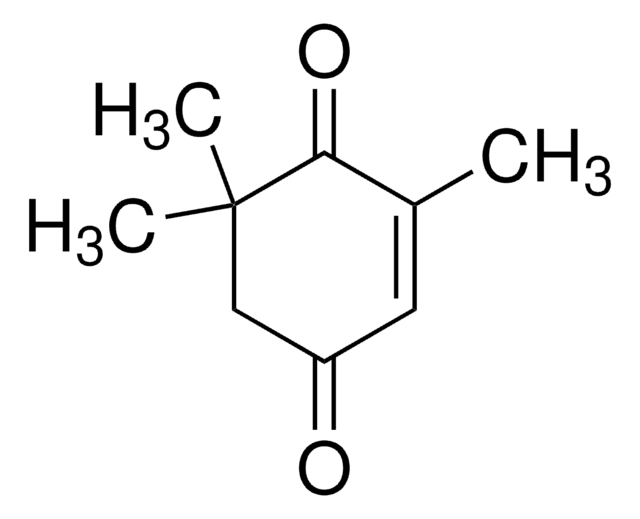
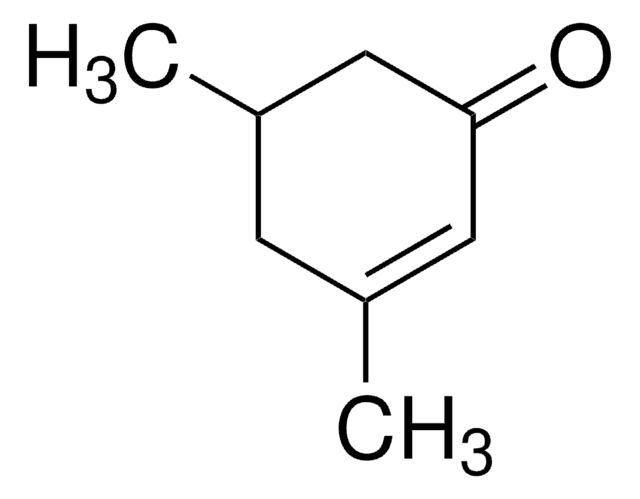
2,6,6-Trimethyl-2-cyclohexene-1,4-dione
98%
Synonym(s):
4-Oxoisophorone, Ketoisophorone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H12O2
CAS Number:
Molecular Weight:
152.19
Beilstein:
2207030
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
refractive index
n20/D 1.491 (lit.)
bp
222 °C (lit.)
92-94 °C/11 mmHg (lit.)
mp
26-28 °C (lit.)
functional group
ketone
SMILES string
CC1=CC(=O)CC(C)(C)C1=O
InChI
1S/C9H12O2/c1-6-4-7(10)5-9(2,3)8(6)11/h4H,5H2,1-3H3
InChI key
AYJXHIDNNLJQDT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2,6,6-Trimethyl-2-cyclohexene-1,4-dione is also known as 4-ketoisophorone and is the major component of saffron spice. It is a cyclic olefin and was reported as a product of the thermal degradation of β-carotene in aqueous suspension.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Description of volatile compounds generated by the degradation of carotenoids in paprika, tomato and marigold oleoresins.
Rios JJ, et al.
Food Chemistry, 106(3), 1145-1153 (2008)
Worldwide market screening of saffron volatile composition.
Maggi L, et al.
Journal of the Science of Food and Agriculture, 89(11), 1950-1954 (2011)
Stefano Raimondi et al.
Journal of biotechnology, 156(4), 279-285 (2011-09-22)
Old yellow enzymes (OYEs, EC 1.6.99.1) are flavin-dependent oxidoreductases that catalyze the stereoselective trans-hydrogenation of the double bond, representing a promising alternative to metal-based catalysis. Bioconversion of ketoisophorone (KIP) by 28 non-conventional yeasts belonging to 16 different species was investigated.
Mohamed-Elamir F Hegazy et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 63(5-6), 403-408 (2008-08-02)
Stereospecific olefin (C=C) and carbonyl (C=O) reduction of the readily available prochiral compound ketoisophorone (2,2,6-trimethyl-2-cyclohexene-1,4-dione) (1) by Marchantia polymorpha and Nicotiana tabacum cell suspension cultures produce the chiral products (6R)-levodione (2), (4R,5S)-4-hydroxy-3,3,5-trimethylcyclohexanone (3), and (4R,6R)-actinol (4) as well as the
Marta Goretti et al.
Bioresource technology, 102(5), 3993-3998 (2011-01-15)
The bioreduction of α,β-unsaturated ketones (ketoisophorone, 2-methyl- and 3-methyl-cyclopentenone) and aldehydes [(S)-(-)-perillaldehyde and α-methyl-cinnamaldehyde] by 23 "non-conventional" yeasts (NCYs) belonging to 21 species of the genera Candida, Cryptococcus, Debaryomyces, Hanseniaspora, Kazachstania, Kluyveromyces, Lindnera, Nakaseomyces, Vanderwaltozyma, and Wickerhamomyces was reported. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service