325430
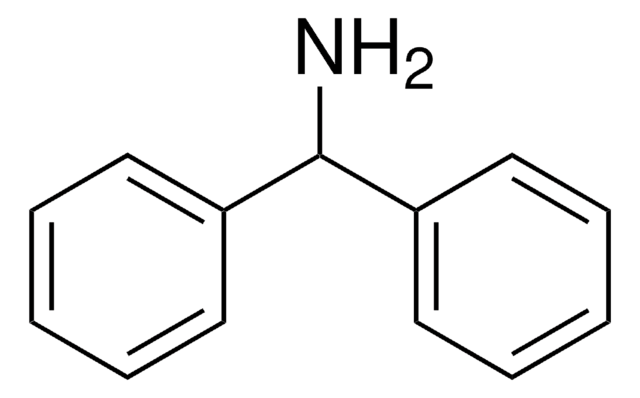
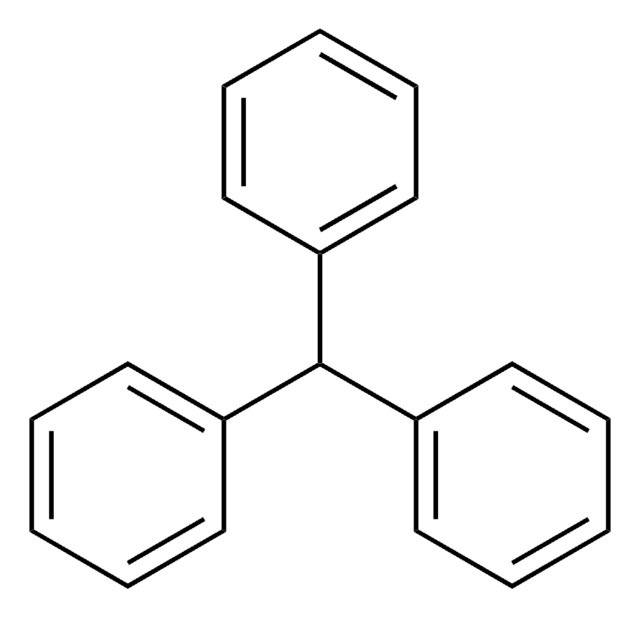
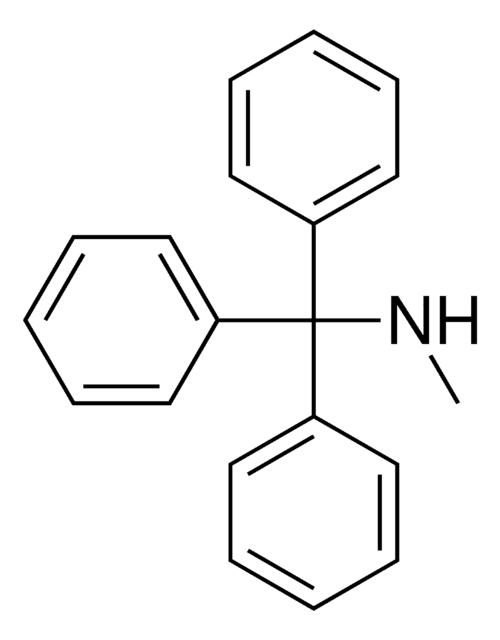
Triphenylmethylamine
99%
Synonym(s):
Tritylamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(C6H5)3CNH2
CAS Number:
Molecular Weight:
259.34
Beilstein:
2113674
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
223 °C/14 mmHg (lit.)
mp
102-104 °C (lit.)
functional group
amine
SMILES string
NC(c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C19H17N/c20-19(16-10-4-1-5-11-16,17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H,20H2
InChI key
BZVJOYBTLHNRDW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Reaction of triphenylmethylamine with borontrifluoride was studied.
Application
Triphenylmethylamine was used to prepare diamondoid porous organic salt. It was also used in preparation of N-tritylated β-aminoalcohols, useful building blocks in organic synthesis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Atsushi Yamamoto et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(9), 3006-3016 (2013-01-12)
A diamondoid porous organic salt (d-POS) composed of 8-hydroxyquinoline-5-sulfonic acid (HQS) and triphenylmethylamine (TPMA) shows reversible structure contraction and expansion ("breathing") in response to guest desorption and adsorption. This flexible structure is designed hierarchically by utilizing two different types of
Reaction of triphenylmethylamines with boron trihalides.
Ronan RJ, et al.
Journal of the American Chemical Society, 93(25), 6811-6814 (1971)
Tritylamine (triphenylmethylamine) in organic synthesis; II. The reaction of tritylamine with oxiranes-synthesis of N-trityl-? aminoalcohols.
Soroka M and Goldeman W.
ARKIVOC (Gainesville, FL, United States), 12, 31-37 (2003)
Grant A McNaughton-Smith et al.
Journal of medicinal chemistry, 51(4), 976-982 (2008-02-01)
Sickle cell disease (SCD) is a hereditary condition characterized by deformation of red blood cells (RBCs). This phenomenon is due to the presence of abnormal hemoglobin that polymerizes upon deoxygenation. This effect is exacerbated when dehydrated RBCs experience a loss
Silke B Bodendiek et al.
Frontiers in pharmacology, 3, 106-106 (2012-06-12)
The paucity of specific pharmacological agents has been a major impediment for delineating the roles of gap junction (GJ) channels formed by connexin proteins in physiology and pathophysiology. Here, we used the selective optimization of side activities (SOSA) approach, which
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service