250376
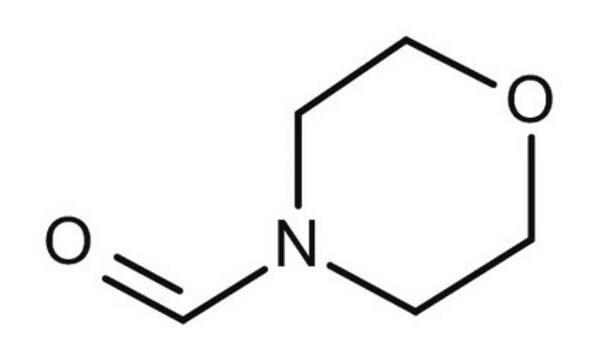
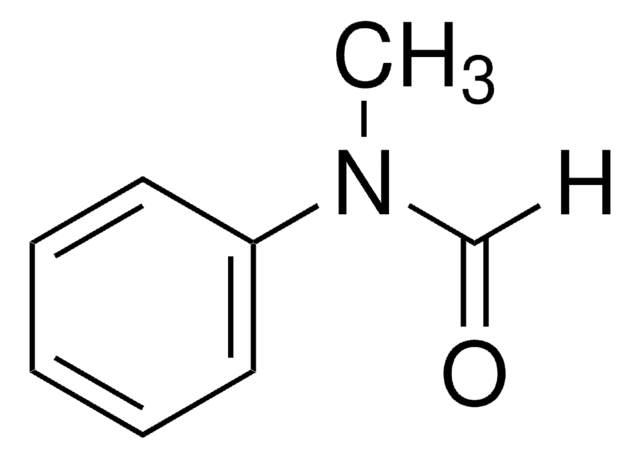
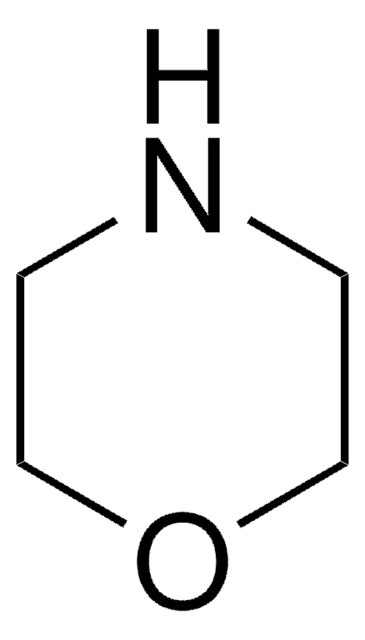
4-Formylmorpholine
99%
Synonym(s):
4-Morpholinecarboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H9NO2
CAS Number:
Molecular Weight:
115.13
Beilstein:
110293
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
refractive index
n20/D 1.485 (lit.)
bp
236-237 °C (lit.)
mp
20-23 °C (lit.)
density
1.145 g/mL at 25 °C (lit.)
functional group
ether
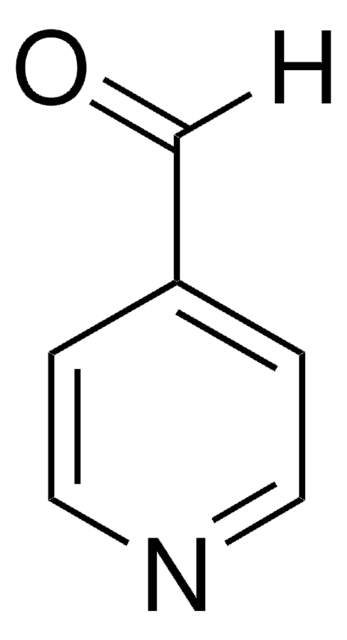
SMILES string
[H]C(=O)N1CCOCC1
InChI
1S/C5H9NO2/c7-5-6-1-3-8-4-2-6/h5H,1-4H2
InChI key
LCEDQNDDFOCWGG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Treatment of 4-formylmorpholine with sulphur tetrafluoride in the presence of potassium fluoride gives 4-(trifluoromethyl)morpholine in excellent yields. 4-Formylmorpholine reacts with series of 2-alkyl-2-cyclohexen-1-ones in the presence of POCl3 to give the corresponding 3-alkyl-2-chloro-5,6-dihydrobenzaldehydes and allylic alcohols (by-product).
Application
4-Formylmorpholine has been used in the preparation of:
- adenine hydrochloride labelled with 14C
- terphenyl dialdehyde
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
244.4 °F - DIN 51758
Flash Point(C)
118 °C - DIN 51758
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
209. A synthesis of adenine labelled with 14C.
Clark VM and Kalckar HM.
Journal of the Chemical Society, 1029-1030 (1950)
Studies in vilsmeier chemistry, V. Vilsmeier reactions of 2-alkyl-2-cyclohexen-1-ones: A novel route to dihydrobenzaldehydes, the formation of allyl alcohols as by-products, and the X-ray crystallographic structure of 3-chloro-2-methyl-2-cyclohexen-1-ol.
Katritzky AR, et al.
Chemische Berichte, 121(5), 999-1003 (1988)
Reaction of tertiary formamides with sulphur tetrafluoride. Direct synthesis of (trifluoromethyl) amines.
Dmowski W and Kaminski M.
Journal of Fluorine Chemistry, 23(3), 207-218 (1983)
Cyclic host having double bonds as bridging units.
Paek K-S and Cram DJ.
Bull. Korean Chem. Soc., 10(6), 569-569 (1989)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service