226351
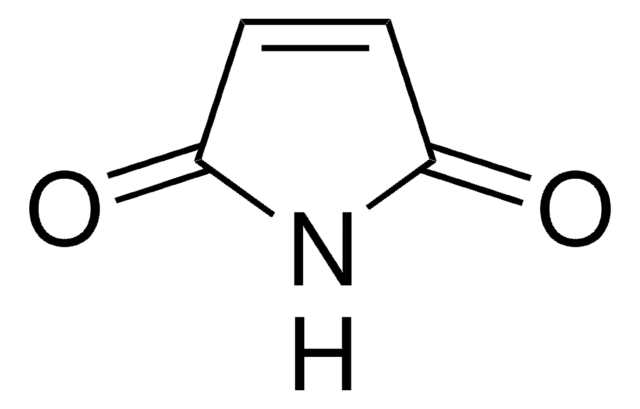
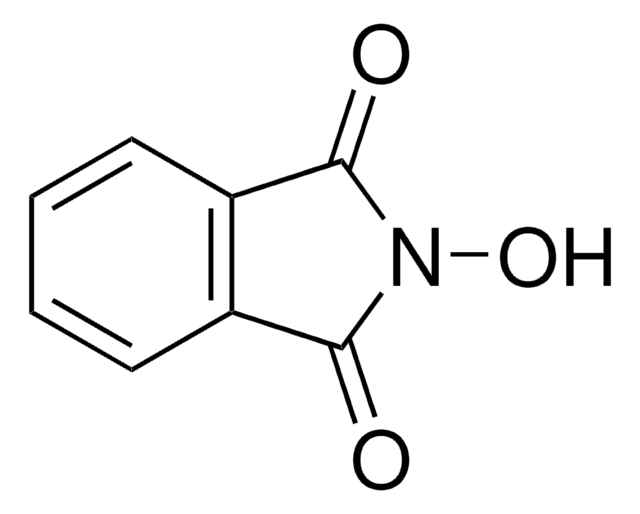
N-Hydroxymaleimide
97%, for peptide synthesis
Synonym(s):
1-Hydroxypyrrole-2,5-dione
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H3NO3
CAS Number:
Molecular Weight:
113.07
Beilstein:
1524594
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
N-Hydroxymaleimide, 97%
Quality Level
Assay
97%
form
solid
reaction suitability
reaction type: Addition Reactions
mp
130-133 °C (dec.) (lit.)
application(s)
peptide synthesis
functional group
imide
maleimide
SMILES string
ON1C(=O)C=CC1=O
InChI
1S/C4H3NO3/c6-3-1-2-4(7)5(3)8/h1-2,8H
InChI key
BUXKULRFRATXSI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-Hydroxymaleimide (NHMI) is an organic compound that is widely used as an oxidizing agent in organic synthesis. It is frequently used to selectively oxidize thiols to disulfides and in the synthesis of α, β-unsaturated carbonyl compounds. It can also act as a Michael acceptor and is used in the synthesis of peptides and proteins.
Application
N-Hydroxymaleimide can be used as an oxidant to generate N-hydroxy anilines in situ, which serve as key intermediates in the selective coupling reaction of N-substituted anilines with hydroxylamine derivatives, for the efficient synthesis of aromatic oximes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jamerson Carneiro de Oliveira et al.
Molecules (Basel, Switzerland), 25(2) (2020-01-16)
The study of Diels-Alder reactions in materials science is of increasing interest. The main reason for that is the potential thermoreversibility of the reaction. Aiming to predict the behavior of a material modified with maleimido and furyl moieties, 1H NMR
Minye Yang et al.
Nanoscale, 10(33), 15865-15874 (2018-08-15)
The detection of mycotoxins in food is urgently needed because they pose a significant threat to public health. In this study, we developed a quantitative detection platform for mycotoxins by integrating multicolor upconversion nanoparticle barcode technology with fluorescence image processing
Yusuke Hibi et al.
Nature communications, 7, 11064-11064 (2016-03-22)
There is a growing interest in sequence-controlled polymers toward advanced functional materials. However, control of side-chain order for vinyl polymers has been lacking feasibility in the field of polymer synthesis because of the inherent feature of chain-growth propagation. Here we
Moon Soo Gil et al.
Journal of controlled release : official journal of the Controlled Release Society, 267, 119-132 (2017-04-17)
Biological drugs are exquisitely tailored components offering the advantages of high specificity and efficacy that are considered safe for treating diseases. Nevertheless, the effectiveness of biological drugs is limited by their inherent short biological half-life and poor stability in vivo.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service