220078
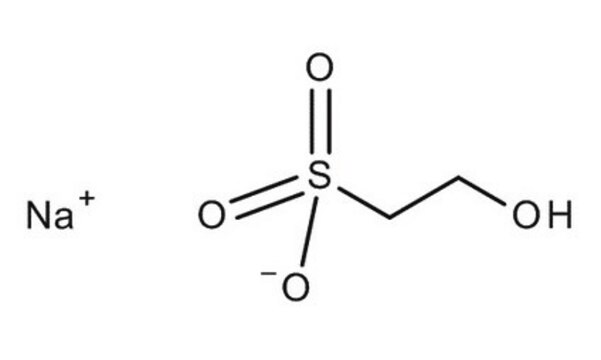
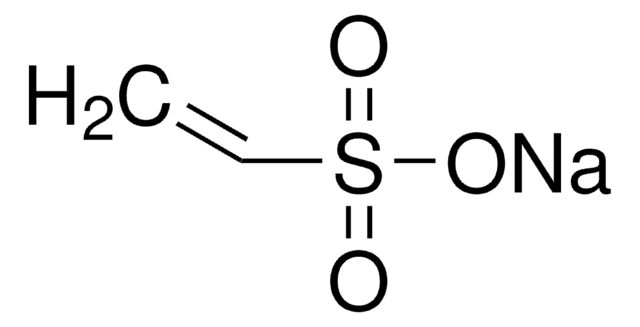
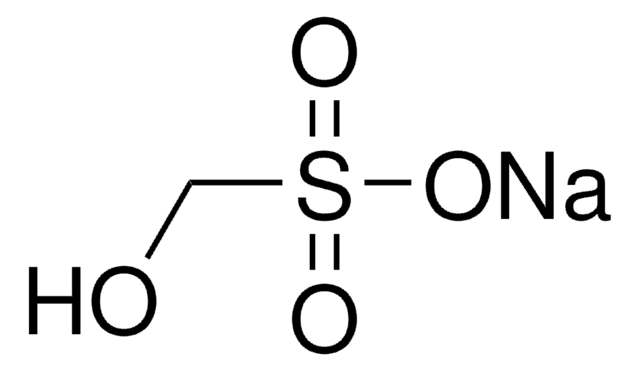
Isethionic acid sodium salt
98%
Synonym(s):
2-Hydroxyethanesulfonic acid sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOCH2CH2SO3Na
CAS Number:
Molecular Weight:
148.11
Beilstein:
3633992
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
191-194 °C (lit.)
functional group
hydroxyl
sulfonic acid
SMILES string
[Na+].OCCS([O-])(=O)=O
InChI
1S/C2H6O4S.Na/c3-1-2-7(4,5)6;/h3H,1-2H2,(H,4,5,6);/q;+1/p-1
InChI key
LADXKQRVAFSPTR-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ping Zhong et al.
PloS one, 6(2), e16970-e16970 (2011-03-09)
Serotonin exerts a powerful influence on neuronal excitability. In this study, we investigated the effects of serotonin on different neuronal populations in prefrontal cortex (PFC), a major area controlling emotion and cognition. Using whole-cell recordings in PFC slices, we found
Kaustubh Sharma et al.
PloS one, 14(7), e0219784-e0219784 (2019-07-12)
Oxytocin is involved in the regulation of social behaviors including parental behaviors in a variety of species. Oxytocin triggers social behaviors by binding to oxytocin receptors (OXTRs) in various parts of the brain. OXTRs are present in the preoptic area
Katharina Styp von Rekowski et al.
Archives of microbiology, 183(5), 325-330 (2005-05-11)
Klebsiella oxytoca TauN1 represents a group of isolates which utilise taurine (2-aminoethanesulfonate) quantitatively as a sole source of combined nitrogen for aerobic growth. During growth, a compound is excreted, which has now been identified as isethionate (2-hydroxyethanesulfonate). An ion-chromatographic separation
M Syringas et al.
Molecular pharmacology, 58(6), 1404-1411 (2000-11-28)
Catecholamine transporters constitute the biological targets for several important drugs, including antidepressants, cocaine, and related compounds. Some information exists about discrete domains of these transporters that are involved in substrate translocation and uptake blockade, but delineation of domains mediating the
Sonja Weinitschke et al.
Applied and environmental microbiology, 76(2), 618-621 (2009-11-26)
Ubiquitous isethionate (2-hydroxyethanesulfonate) is dissimilated by diverse bacteria. Growth of Cupriavidus necator H16 with isethionate was observed, as was inducible membrane-bound isethionate dehydrogenase (IseJ) and inducible transcription of the genes predicted to encode IseJ and a transporter (IseU). Biodiversity in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service