All Photos(1)
About This Item
Empirical Formula (Hill Notation):
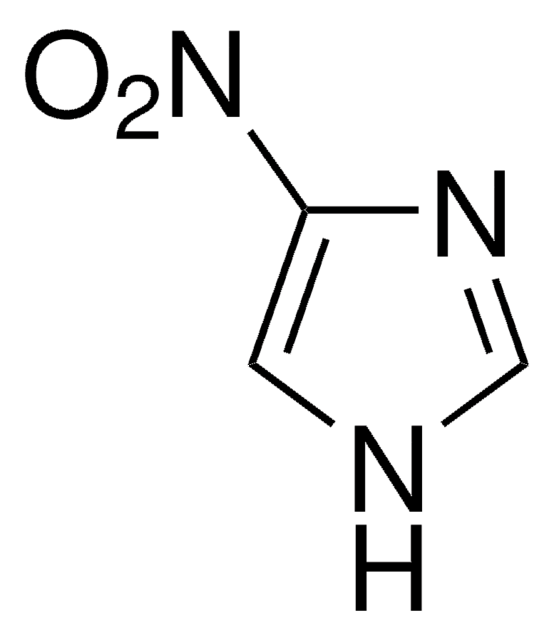
C3H3N3O2
CAS Number:
Molecular Weight:
113.07
Beilstein:
116444
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
287 °C (dec.) (lit.)
functional group
nitro
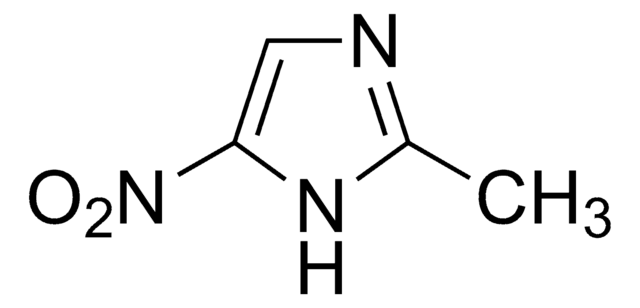
SMILES string
[O-][N+](=O)c1ncc[nH]1
InChI
1S/C3H3N3O2/c7-6(8)3-4-1-2-5-3/h1-2H,(H,4,5)
InChI key
YZEUHQHUFTYLPH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Nitroimidazole is a natural antibiotic.
Application
2-Nitroimidazole was used in the synthesis of:
- tert-butyl 2-(2-nitro-1H-imidazol-1-yl)ethylcarbamate
- 1-(2-(tert-butyldimethylsilyloxy)ethyl)-2-nitro-1H-imidazole
- 2-fluoro-N-(2-(2-nitro-1H-imidazol-1-yl)ethyl)acetamide
- 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl 2-fluoroacetate
- radiolabeling precursors - the bromo substituted analogs
- nitroimidazole substituted boronic acids, precursors for imaging hypoxic tissue
- potential site-selective radiosensitizers for estrogen receptor-rich tumors
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Analysis Note
solubility :
50 mg/mL in NH4OH : soluble, clear, green-yellow, to very dark green-yellow to very dark yellow
50 mg/mL in NH4OH : soluble, clear, green-yellow, to very dark green-yellow to very dark yellow
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alane Beatriz Vermelho et al.
Journal of enzyme inhibition and medicinal chemistry, 33(1), 139-146 (2017-12-02)
Sulfonamide carbonic anhydrase (CA, EC 4.2.1.1) inhibitors targeting the α-class enzyme from the protozoan pathogen Trypanosoma cruzi, responsible of Chagas disease, were recently reported. Although many such derivatives showed low nanomolar activity in vitro, they were inefficient anti-T. cruzi agents
Kazuki Yoshihara et al.
Chembiochem : a European journal of chemical biology, 18(16), 1650-1658 (2017-05-16)
The use of DNA aggregates could be a promising strategy for the molecular imaging of biological functions. Herein, phosphorescent oligodeoxynucleotides were designed with the aim of visualizing oxygen fluctuation in tumor cells. DNA-ruthenium conjugates (DRCs) that consisted of oligodeoxynucleotides, a
J. Chem. Res. Synop., 92-92 (1993)
Yi Qu et al.
Environmental microbiology, 13(4), 1010-1017 (2011-01-20)
Antibiotic resistance in pathogens can be mediated by catabolic enzymes thought to originate from soil bacteria, but the physiological functions and evolutionary origins of the enzymes in natural ecosystems are poorly understood. 2-Nitroimidazole (2NI) is a natural antibiotic and an
Christin Glowa et al.
Radiation oncology (London, England), 12(1), 174-174 (2017-11-11)
To summarize the research activities of the "clinical research group heavy ion therapy", funded by the German Research Foundation (DFG, KFO 214), on the impact of intrinsic tumor characteristics (grading, hypoxia) on local tumor control after carbon ( Three sublines
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service