All Photos(3)
About This Item
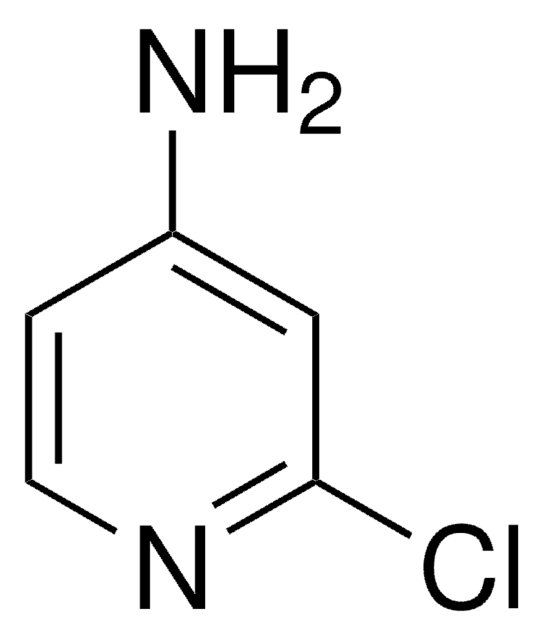
Empirical Formula (Hill Notation):
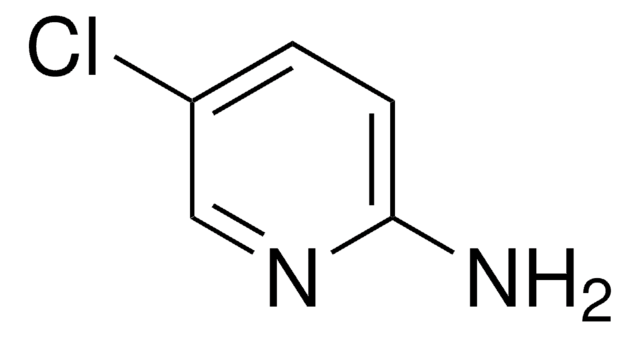
C5H5ClN2
CAS Number:
Molecular Weight:
128.56
Beilstein:
108891
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
81-83 °C (lit.)
SMILES string
Nc1ccc(Cl)nc1
InChI
1S/C5H5ClN2/c6-5-2-1-4(7)3-8-5/h1-3H,7H2
InChI key
QAJYCQZQLVENRZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
5-Amino-2-chloropyridine undergoes Suzuki-Miyaura coupling with sterically hindered 2,6-dimethylphenylboronic acid. It undergoes facile temperature dependent displacement of chloride by bromide via Sandmeyer reaction to yield 2,5-dibromopyridine.
Application
5-Amino-2-chloropyridine was used in the synthesis of [2H5]2-amino-l-methyl-6-phenylimidazo[4,5-b]pyridine. It was used in identification and evaluation of molecularly imprinted polymers for the selective removal of potentially genotoxic aminopyridine impurities from pharmaceuticals.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A M Lynch et al.
Cancer research, 52(22), 6216-6223 (1992-11-15)
During the cooking of beef, the genotoxic heterocyclic aromatic amines 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQx), and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) are formed. Little is known about the fate of these compounds in humans or the factors affecting it. We have developed assays based
Unexpected Displacements of Chloride by Bromide Found During Sandmeyer Reactions of 3-or 5-Amino-2-chloropyridines.
Krapcho AP and Haydar SN.
Heterocyclic Communications, 4(4), 291-292 (1998)
Fast identification of selective resins for removal of genotoxic aminopyridine impurities via screening of molecularly imprinted polymer libraries.
Kecili R, et al. et al.
Journal of Chromatography A (2014)
A highly active catalyst for Suzuki-Miyaura cross-coupling reactions of heteroaryl compounds.
Kelvin L Billingsley et al.
Angewandte Chemie (International ed. in English), 45(21), 3484-3488 (2006-04-28)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service