All Photos(3)
About This Item
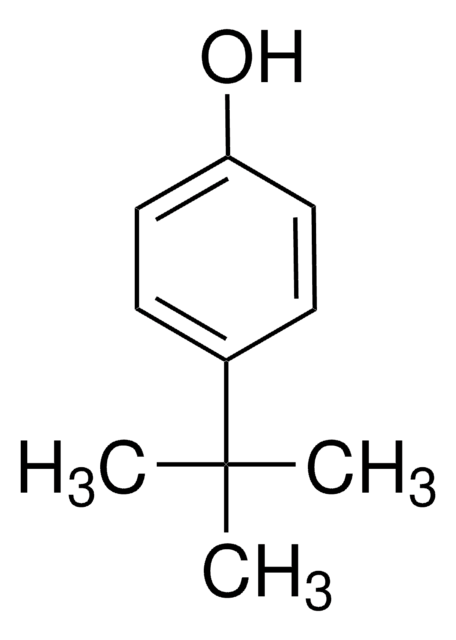
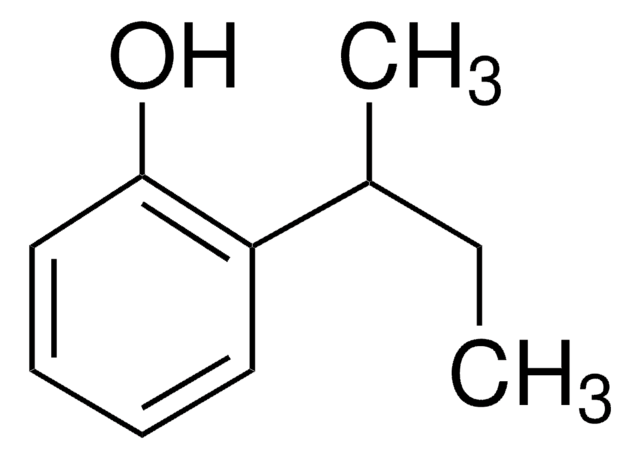
Linear Formula:
(CH3)2CHC6H4OH
CAS Number:
Molecular Weight:
136.19
Beilstein:
1363564
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
>1 (vs air)
Assay
98%
form
crystals
bp
212-213 °C (lit.)
mp
59-61 °C (lit.)
SMILES string
CC(C)c1ccc(O)cc1
InChI
1S/C9H12O/c1-7(2)8-3-5-9(10)6-4-8/h3-7,10H,1-2H3
InChI key
YQUQWHNMBPIWGK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Sonochemical degradation of 4-Isopropylphenol has been studied.
Application
4-Isopropylphenol was used in the synthesis of vinyl compounds.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - Pensky-Martens closed cup
Flash Point(C)
110 °C - Pensky-Martens closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mahdi Chiha et al.
Ultrasonics sonochemistry, 17(5), 773-782 (2010-04-15)
Sonochemical degradation of phenol (Ph), 4-isopropylphenol (4-IPP) and Rhodamine B (RhB) in aqueous solutions was investigated for a large range of initial concentrations in order to analyze the reaction kinetics. The initial rates of substrate degradation and H(2)O(2) formation as
Frédéric L P Gabriel et al.
Chemistry & biodiversity, 4(9), 2123-2137 (2007-09-25)
Sphingobium xenophagum Bayram is capable of metabolizing 4-alkoxyphenols and endocrine disruptive alpha-quaternary 4-nonylphenols by an ipso-substitution mechanism that involves ring hydroxylation at the site of the substituent. Here, we show that Bayram's ipso-hydroxylating activity was able to transform also bisphenol
David J Hopper et al.
Applied and environmental microbiology, 69(6), 3650-3652 (2003-06-06)
4-ethylphenol methylenehydroxylase from Pseudomonas putida JD1 acts by dehydrogenation of its substrate to give a quinone methide, which is then hydrated to an alcohol. It was shown to be active with a range of 4-alkylphenols as substrates. 4-n-propylphenol, 4-n-butylphenol, chavicol
D C Thompson et al.
Chemical research in toxicology, 8(1), 55-60 (1995-01-01)
The oxidative metabolism and toxicity of the para isomers of methylphenol (cresol), ethylphenol, and isopropylphenol were studied using male Sprague-Dawley rat liver microsomes and precision-cut liver slices. Reactive intermediates from each compound were trapped using radiolabeled glutathione and were detected
Shogo Kumagai et al.
Scientific reports, 8(1), 13994-13994 (2018-09-20)
The pyrolysis of bisphenol A (BPA), an essential process ingredient used in industry and many everyday life products, helps produce low-industrial-demand chemicals such as isopropenyl- and isopropyl-phenols (IPP and iPrP). In this study, tandem micro-reactor gas chromatography/mass spectrometry combined with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service