All Photos(1)
About This Item
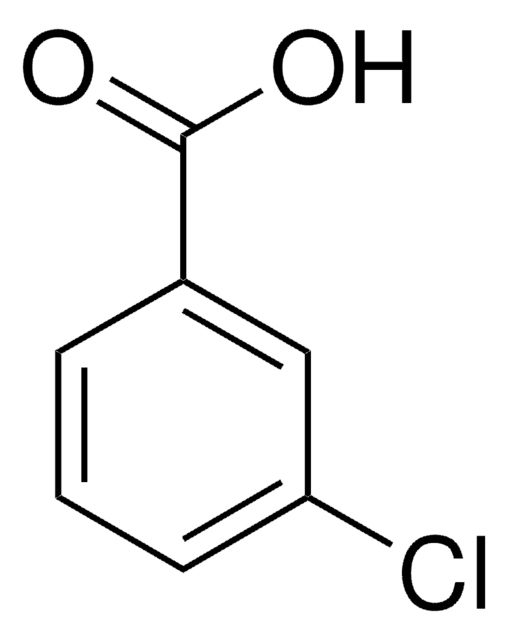
Linear Formula:
IC6H4CO2H
CAS Number:
Molecular Weight:
248.02
Beilstein:
971088
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
185-187 °C (lit.)
functional group
carboxylic acid
iodo
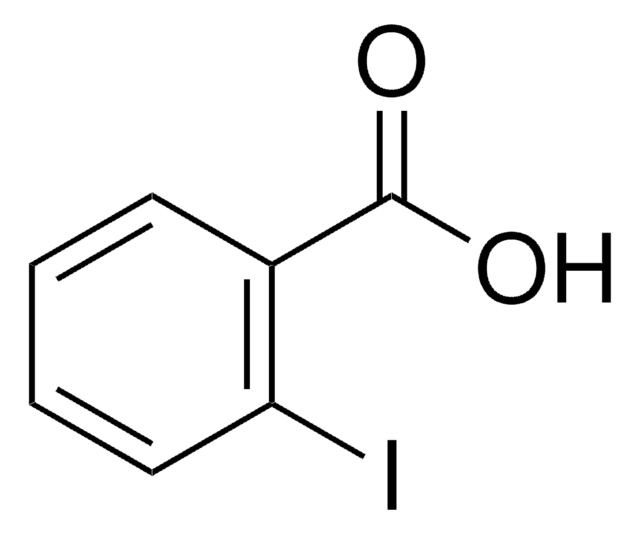
SMILES string
OC(=O)c1cccc(I)c1
InChI
1S/C7H5IO2/c8-6-3-1-2-5(4-6)7(9)10/h1-4H,(H,9,10)
InChI key
KVBWBCRPWVKFQT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Iodobenzoic acid is added as UV absorbing background electrolyte in separation of uncharged cyclodextrins and their derivatives by capillary electrophoresis.
Application
3-Iodobenzoic acid was used in solid phase synthesis of γ-turn mimetic library.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A γ-Turn Mimetic Library: Development and Production.
Kocis P, et al.
High-Throughput Synthesis: Principles and Practices, 65-65 (2010)
M Pumera et al.
Fresenius' journal of analytical chemistry, 369(7-8), 666-669 (2001-05-24)
A fast and simple capillary electrophoretic method suitable for the determination of native alpha-, beta-, gamma-cyclodextrins, their randomly substituted tert-butyl derivatives (average degree of substitution 3.8-4.4), heptakis (2,6-di-O-methyl)- and heptakis (2,3,6-tri-O-methyl)-beta-cyclodextrin was developed. Naphthyl-2-sulfonic acid (2-NSA), 3-iodobenzoic acid (3-IBA) and
Negative inotropic effects of Na-salicylate and three congeners on the guinea-pig Langendorff heart.
H Brasch
Archives internationales de pharmacodynamie et de therapie, 262(2), 242-249 (1983-04-01)
In guinea-pig Langendorff hearts, Na-salicylate (1.9, 3.8 and 7.6 mmol/l) concentration-dependently reduced the contractile force (--9.1, --51.0 and --75.1%, respectively) and the coronary resistance. The influence of the uncoupling agent 2.4-dinitrophenol (0.02 mmol/l) was comparable to that of the largest
Pablo Wessig et al.
Molecules (Basel, Switzerland), 18(1), 1314-1324 (2013-01-23)
Various 1,6- and 1,8-naphthalenophanes were synthesized by using the Photo-Dehydro-Diels-Alder (PDDA) reaction of bis-ynones. These compounds are easily accessible from ω-(3-iodophenyl)carboxylic acids in three steps. The obtained naphthalenophanes are axially chiral and the activation barrier for the atropisomerization could be
G Vaidyanathan et al.
Bioconjugate chemistry, 1(6), 387-393 (1990-11-01)
We have previously shown that use of N-succinimidyl 3-iodobenzoate (SIB) for radioiodination of monoclonal antibodies (MAbs) decreases the loss of radioiodine in vivo compared to MAbs labeled by using conventional methods. Herein, the synthesis of N-succinimidyl 2,4-dimethoxy-3-(trialkylstannyl)benzoates (alkyl = Me
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service