132888
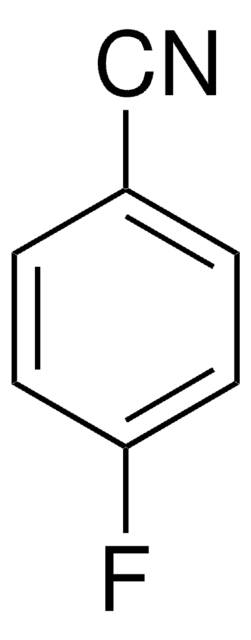
2-Chloro-4′-fluoroacetophenone
99%
Synonym(s):
ω-Chloro-4-fluoroacetophenone, 4-Fluorophenacyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
FC6H4COCH2Cl
CAS Number:
Molecular Weight:
172.58
Beilstein:
637860
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
47-50 °C (lit.)
functional group
chloro
SMILES string
Fc1ccc(cc1)C(=O)CCl
InChI
1S/C8H6ClFO/c9-5-8(11)6-1-3-7(10)4-2-6/h1-4H,5H2
InChI key
UJZWJOQRSMOFMA-UHFFFAOYSA-N
Gene Information
human ... PTPN6(5777)
Looking for similar products? Visit Product Comparison Guide
General description
2-Chloro-4′-fluoroacetophenone on condensation with N-methyl-4-(methylthio)benzamidine yields 1-methyl-2-(4-methylthio)phenyl-4-(4-fluoro)phenylimidazole.
Application
2-Chloro-4′-fluoroacetophenone was used in the synthesis of S-(phenacyl)glutathiones and 2-[3-(4-chlorophenyl)-3-hydroxy-8-azabicyclo[3.2.1]oct-8-yl]-1-(4-fluorophenyl)ethanone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
2, 4, 5-Triarylimidazole inhibitors of IL-1 biosynthesis.
Gallagher TF, et al.
Bioorganic & Medicinal Chemistry Letters, 5(11), 1171-1176 (1995)
Allergic contact dermatitis from 3-(alpha-methoxy) methylenebenzofuran-2(3H)-one (MBF) and alpha-chloro-4-fluoroacetophenone (CFAP) in chemical process workers.
M J Boffa et al.
Contact dermatitis, 34(6), 434-435 (1996-06-01)
Philip G Board et al.
Chemical research in toxicology, 20(1), 149-154 (2007-01-18)
S-(Phenacyl)glutathione reductase (SPG-R) plays a significant role in the biotransformation of reactive alpha-haloketones to nontoxic acetophenones. Comparison of the apparent subunit size, amino acid composition, and catalysis of the reduction of S-(phenacyl)glutathiones indicated that a previously described rat SPG-R (Kitada
Synthesis and evaluation of ligands for D2-like receptors: the role of common pharmacophoric groups.
Donald M N Sikazwe et al.
Bioorganic & medicinal chemistry, 17(4), 1716-1723 (2009-01-22)
Arylcycloalkylamines, such as phenyl piperidines and piperazines and their arylalkyl substituents, constitute pharmacophoric groups exemplified in several antipsychotic agents. A review of previous reports indicates that arylalkyl substituents can improve the potency and selectivity of the binding affinity at D(2)-like
Justin D Smith et al.
Nature communications, 10(1), 1837-1837 (2019-04-25)
Photocatalytic polymers offer an alternative to prevailing organometallics and nanomaterials, and they may benefit from polymer-mediated catalytic and material enhancements. MPC-1, a polymer photoredox catalyst reported herein, exhibits enhanced catalytic activity arising from charge transfer states (CTSs) between its two
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service