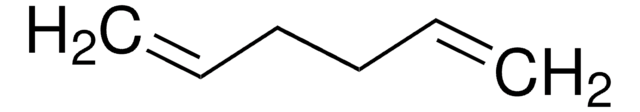
125415
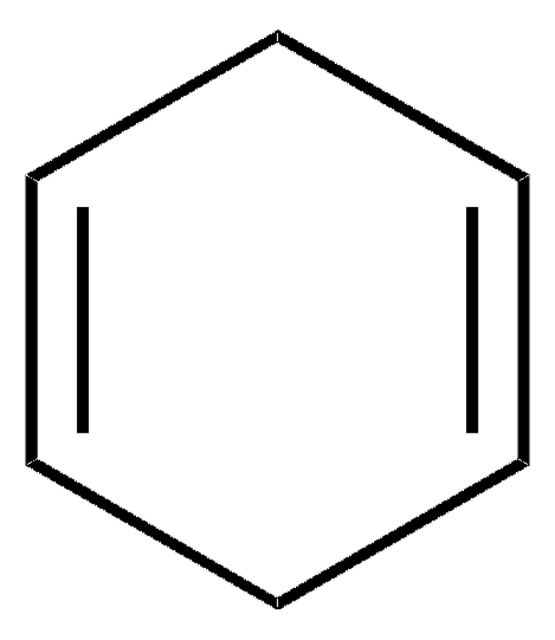
1,4-Cyclohexadiene
97%
Synonym(s):
1,4-Dihydrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H8
CAS Number:
Molecular Weight:
80.13
Beilstein:
1900733
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
contains
~0.1% hydroquinone as stabilizer
impurities
3% benzene
refractive index
n20/D 1.472 (lit.)
bp
88-89 °C (lit.)
density
0.847 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1C=CCC=C1
InChI
1S/C6H8/c1-2-4-6-5-3-1/h1-2,5-6H,3-4H2
InChI key
UVJHQYIOXKWHFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,4-Cyclohexadiene is an effective hydrogen donor for catalytic hydrogenation reactions. It can rapidly replace benzyl groups of N-benzyloxycarbamates, benzyl esters, benzyl ethers and benzyl amines with hydrogen. It forms benzene at elevated temperatures in the presence of a ruthenium(II)-triphenylphosphine catalyst.
Application
1,4-Cyclohexadiene (1,4-CHD) was used to study the formation of parent ion from heavy fragmentation of 1,4-CHD on irradiation with a high-intensity laser pulse.
Useful for the reduction of radical intermediates formed in electron-transfer mediated ring-opening reactions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1A - Flam. Liq. 2 - Muta. 1B - STOT RE 2
Target Organs
Blood
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
19.4 °F - closed cup
Flash Point(C)
-7 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rapid removal of protecting groups from peptides by catalytic transfer hydrogenation with 1, 4-cyclohexadiene.
Felix AM,et al.
The Journal of Organic Chemistry, 43(21), 4194-4196 (1978)
A key factor in parent and fragment ion formation on irradiation with an intense femtosecond laser pulse.
Harada H, et al.
Chemical Physics Letters, 342(5), 563-570 (2001)
Organometallics, 25, 5456-5456 (2006)
Kazutada Ikeuchi et al.
Organic letters, 14(23), 6016-6019 (2012-11-15)
Asymmetric bromolactonization of prochiral cyclohexadiene derivatives with N-bromosuccimide proceeded in the presence of (DHQD)(2)PHAL as a chiral catalyst to afford the corresponding bromolactones with up to 93% ee. This reaction was also applicable to the kinetic resolution of a racemic
Yide Gao et al.
The journal of physical chemistry. A, 113(25), 6955-6963 (2009-06-06)
A quantitative understanding of the thermochemistry of cyclohexadienyl radical and 1,4-cyclohexadiene is beneficial for diverse areas of chemistry. Given the interest in these two species, it is surprising that more detailed thermodynamic data concerning the homolytic C-H bond enthalpies of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service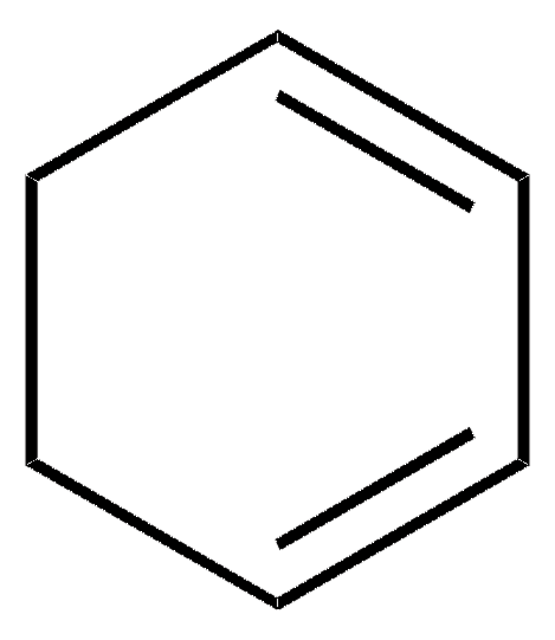

![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)