123056
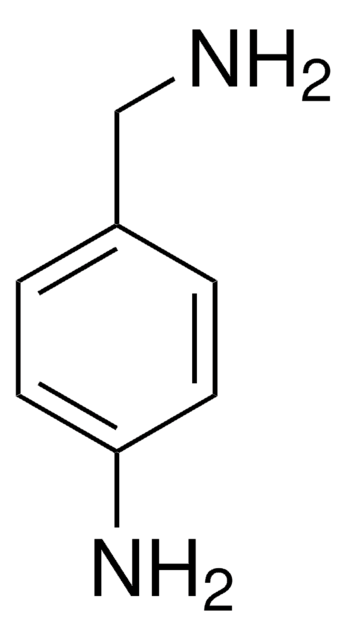
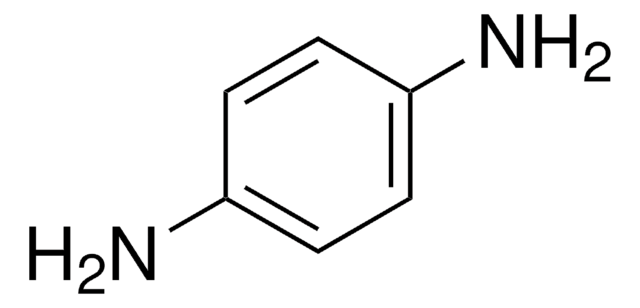
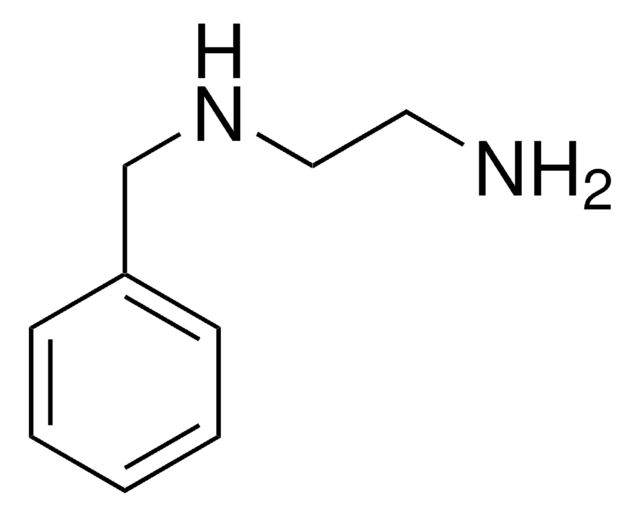
4-(2-Aminoethyl)aniline
97%
Synonym(s):
4-Aminophenethylamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NC6H4CH2CH2NH2
CAS Number:
Molecular Weight:
136.19
Beilstein:
1099913
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.591 (lit.)
bp
103 °C/0.3 mmHg (lit.)
mp
28-31 °C (lit.)
density
1.034 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
NCCc1ccc(N)cc1
InChI
1S/C8H12N2/c9-6-5-7-1-3-8(10)4-2-7/h1-4H,5-6,9-10H2
InChI key
LNPMZQXEPNWCMG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-(2-Aminoethyl)aniline undergoes coupling with carbohydrates by reductive amination to yield modified carbohydrates.
Application
4-(2-Aminoethyl)aniline has been used in chemical modification of silk fibroin to tailor the overall hydrophilicity and structure of silk. It has been used as reagent in polycondensation reactions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mihaela Badea et al.
Biosensors & bioelectronics, 18(5-6), 689-698 (2003-04-23)
Glucose oxidase, lactate oxidase, L-aminoacid oxidase and alcohol oxidase were immobilised on new films based on 2,6-dihydroxynaphthalene (2,6-DHN) copolymerised with 2-(4-aminophenyl)-ethylamine (AP-EA) onto the Pt electrodes. The electropolymerisation was performed by cyclic voltammetry. Different scan rates and scan potential ranges
Mitsuo Okada et al.
Plant & cell physiology, 43(5), 505-512 (2002-06-01)
Binding experiments as well as affinity labeling with an (125)I-labeled 2-(4-aminophenyl)ethylamino derivative of N-acetylchitooctaose revealed the presence of high-affinity binding sites/proteins for N-acetylchitooligosaccharide elicitor in the plasma membrane preparation from suspension-cultured carrot cells, barley cells and wheat leaves. Their binding
Chem. Abstr., 116, 106932j-106932j (1992)
H D Grimmecke et al.
Glycoconjugate journal, 15(6), 555-562 (1999-01-09)
Reductive amination of 3-deoxy-D-manno-octulosonic acid (Kdo) with allylamine (AIIN) or 2-(4-aminophenyl)ethylamine (APEA) yields epimer pairs of 2-N-allylamino and 2-N-[2-(4-aminophenyl)ethylamino]-2,3-dideoxy-D-glycero-D-galacto- and -2,3-dideoxy-D-glycero-D-talo-octonic acid. The yields were 50-60% due to reduction of Kdo to the respective polyols as side reaction products. Mass
An improved method for the preparation of derivatives of reducing oligosaccharide with 2-(4-aminophenyl)ethylamine.
L H Semprevivo
Carbohydrate research, 177, 222-227 (1988-06-15)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service