103373
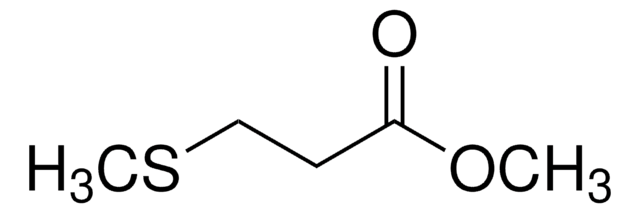
Methyl 3-(methylthio)propionate
98%
Synonym(s):
Methyl 3-(methylmercapto)propionate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3SCH2CH2COOCH3
CAS Number:
Molecular Weight:
134.20
Beilstein:
1745077
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.465 (lit.)
bp
74-75 °C/13 mmHg (lit.)
density
1.077 g/mL at 25 °C (lit.)
functional group
ester
thioether
SMILES string
COC(=O)CCSC
InChI
1S/C5H10O2S/c1-7-5(6)3-4-8-2/h3-4H2,1-2H3
InChI key
DMMJVMYCBULSIS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methyl 3-(methylthio)propionate is the major ester present in the flavour profile of juices made from fresh-cut pineapple fruits.
Application
Methyl 3-(methylthio)propionate was used to prepare 3-methiolpropionate(MMPA) to study the demethylation of Dimethylsulfoniopropionate and MMPA to 3-mercaptopropionate by aerobic marine bacterium. Methyl 3-(methylthio)propionate was used to prepare 3-(Methylthio)propionic acid to examine the methionine catabolism by Lactococci by 13C Nuclear Magnetic Resonance and Gas Chromatography.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
161.6 °F - closed cup
Flash Point(C)
72 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Gao et al.
Applied and environmental microbiology, 64(12), 4670-4675 (1998-12-03)
Formation of methanethiol from methionine is widely believed to play a significant role in development of cheddar cheese flavor. However, the catabolism of methionine by cheese-related microorganisms has not been well characterized. Two independent methionine catabolic pathways are believed to
P T Visscher et al.
Applied and environmental microbiology, 60(12), 4617-4619 (1994-12-01)
A bacterium, strain BIS-6, that grew aerobically on dimethylsulfoniopropionate (DMSP) was isolated from an intertidal mud sample. Strain BIS-6 quantitatively demethylated DMSP and 3-methiolpropionate to 3-mercaptopropionate. Strain BIS-6 was a versatile methylotroph growing on the osmolytes DMSP and glycine betaine
Aroma profiles of pineapple fruit (Ananas comosus [L.] Merr.) and pineapple products.
Elss S, et al.
Food Sci. Technol., 38(3), 263-274 (2005)
He Fu et al.
Journal of bacteriology, 197(8), 1515-1524 (2015-02-19)
Methanosarcina acetivorans uses a variety of methylated sulfur compounds as carbon and energy sources. Previous studies implicated the mtsD, mtsF, and mtsH genes in catabolism of dimethylsulfide, but the genes required for use of other methylsulfides have yet to be
Yuwadee Ackarabanpojoue et al.
Journal of food science, 80(5), E998-1004 (2015-04-02)
This study aimed at investigating the effect of nitrate removal from pineapple juice by electrodialysis (ED) on selected properties of the ED-treated juice. Single-strength pineapple juice with reduced pulp content was treated by ED to reduce the nitrate concentration to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service