GenElute™ Plant Genomic DNA Miniprep Kit Protocol (G2N10, G2N70, G2N350)
Product Description
Several micrograms of DNA can be obtained from up to 100 mg of fresh tissue or 10 mg of freeze-dried material in less than an hour. The purified DNA is greater than 20 kb in length and can be used in sensitive downstream applications such as restriction endonuclease digests and PCR amplification.
Equipment and Reagents Required but Not Provided
- Small mortar and pestle
- Liquid nitrogen
- Microcentrifuge tubes
- Microcentrifuge*
- RNase A Solution, Catalog No. R4642
- Ethanol (95–100%), Catalog Nos. E7148, E7023, or 459836
- Molecular Biology Reagent Water, Catalog No. W4502
- 65 °C water bath
* Note: To ensure proper fit of all tubes, a 24-place rotor is recommended. If you are using a 36-place rotor, we recommend using every other place for proper tube fit.
Storage and Stability
Store the kit at room temperature. If any kit reagent forms a precipitate upon storage, warm at 55–65 °C until the precipitate dissolves.
Preparation Instructions
Thoroughly Mix Reagents
Examine reagents for precipitation. If any reagent forms a precipitate, warm at 55-65 °C until the precipitate dissolves and allow to cool to room temperature before use.Preheat the Water Bath to 65 °C
Wash Solution
Dilute the Wash Solution Concentrate with 9.5 mL (10 prep package), 72 mL (70 prep package), or 330 mL (350 prep package) of 95-100% ethanol. After each use, tightly cap the diluted wash solution to prevent the evaporation of ethanol.Preheat the Elution Solution to 65 °C

Procedure
1. Disrupt Cells
Grind plant tissue into a fine powder in liquid nitrogen using a mortar and pestle. Transfer up to 100 mg of the powder to a microcentrifuge tube. Keep the sample on ice for immediate use or freeze as –70 °C.
2. Lyse Cells
Add 350 µL of Lysis Solution [Part A] and 50 µL of Lysis Solution [Part B] to the tube; thoroughly mix by vortexing and inverting. A white precipitate will form upon the addition of Lysis Solution [Part B]. Incubate the mixture at 65 °C for 10 minutes with occasional inversion to dissolve the precipitate.
Optional digest with RNase: This kit is designed to selectively isolate large DNA. If preparations are found to be contaminated with RNA, RNase A (not supplied) can be used to digest the RNA. Add 50 units of RNase A to the lysis mixture just prior to incubation at 65 °C.
3. Precipitate Debris
Add 130 µL of Precipitation Solution to the mixture; mix completely by inversion and place the sample on ice for 5 minutes. Centrifuge the sample at maximum speed (12,000–16,000 x g) for 5 minutes to pellet the cellular debris, proteins, and polysaccharides.
4. Filter Debris
Carefully pipette the supernatant from step 3 onto a GenElute filtration column (blue insert with a 2 mL collection tube). Centrifuge at maximum speed for 1 minute. This removes any cellular debris not removed in step 3. Discard the filtration column, but retain the collection tube.
5. Prepare for Binding
Add 700 µL of Binding Solution directly to the flowthrough liquid from step 4. Mix thoroughly by inversion.
6. Prepare Binding Column
Insert a GenElute Miniprep Binding Column (with a red o-ring) into a provided microcentrifuge tube, if not already assembled. Add 500 µL of the Column Preparation Solution to each miniprep column and centrifuge at 12,000 x g for 30 seconds to 1 minute. Discard the flow-through liquid
Note: The Column Preparation Solution maximizes binding of DNA to the membrane resulting in more consistent yields.
7. Load Lysate
Carefully pipette 700 µL of the mixture from step 5 onto the column prepared in step 6 and centrifuge at maximum speed for 1 minute. Discard the flow-through liquid; retain the collection tube. Return the column to the collection tube. Apply the remaining lysate from step 5 onto the column. Repeat the centrifugation as above and discard the flow-through liquid and collection tube.
8. First Column Wash
Prior to first time use, be sure to add ethanol to the Wash Solution Concentrate. Place the binding column into a fresh 2 mL collection tube and apply 500 µL of the diluted Wash Solution to the column. Centrifuge at maximum speed for 1 minute. Discard the flow-through liquid, but retain the collection tube.
9. Second Column Wash
Apply another 500 µL of diluted Wash Solution to the column and centrifuge at maximum speed for 3 minutes to dry the column. Do not allow the flow-through liquid to contact the column; wipe off any fluid that adheres to the outside of the column.
10. Elute DNA
Transfer the binding column to a fresh 2 mL collection tube. Apply 100 µL of pre-warmed (65 °C) Elution Solution to the column and centrifuge at maximum speed for 1 minute. Repeat the elution. Do not allow the flow-through liquid to contact the column. Eluates may be collected in the same collection tube. Alternatively, a second collection tube may be used for the second elution to prevent dilution of the first eluate.
The eluate contains pure genomic DNA. For short-term storage of DNA, 2–8 °C is recommended. For long-term storage of DNA, –20 °C is recommended. Avoid freezing and thawing, which causes breaks in the DNA strand. Elution Solution will help stabilize the DNA at these temperatures.
DNA Precipitation (Optional)
The GenElute Blood Genomic DNA Kit is designed so that the DNA always remains in solution, which avoids resuspension issues. However, if it is necessary to concentrate the DNA, ethanol precipitation in the presence of sodium acetate is recommended.1
Alternative Disruption Procedures
The extraction of nucleic acid from plant tissue is complicated by the tough cell wall that surrounds most plant cells as well as the fibrous nature of many species. Several methods exist for the disruption of plant material. One of the most effective and commonly used methods is to grind the tissue in liquid nitrogen with a mortar and pestle. The GenElute Plant Genomic DNA Miniprep Kit was developed based on this efficient method of disruption. However, other disruption techniques can be substituted for step 1 of the Procedure.
Good yields of high molecular weight DNA can also be obtained from freeze-dried tissue. Dried tissue should be ground into a fine powder with a mortar and pestle; up to 20 mg of this powder can be used in a single DNA preparation. Liquid nitrogen is not necessary during the grinding of freeze-dried tissue. After grinding the tissue into a powder, follow the Procedure beginning with step 2.
Results
Determine the concentration and purity of the plant DNA by spectrophotometric analysis and agarose gel electrophoresis. The ratio of absorbance at 260 nm to 280 nm (A260/A280) should be 1.7 to 1.9. The size and quality of the DNA can be determined by agarose gel electrophoresis or pulsed field electrophoresis.
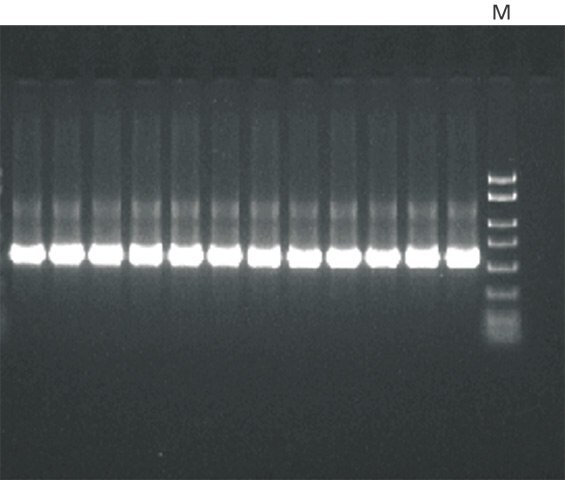
Figure 1.PCR amplification of a 500 bp product isolated from genomic DNA. Genomic DNA from soybean leaves was purified using the GenElute™ Plant Genomic DNA Miniprep Kit. A 5 μL aliquot of eluate was used as template in a 20 μL total PCR reaction for 30 cycles. A 5 μL aliquot of each PCR reaction was resolved on a 2% precast agarose gel (Cat. No. P5722). The PCR marker (M) used (Cat. No. P9577) ranged from 50 bp to 2 kb.

Figure 2.Genomic DNA from various plant species isolated with GenElute™ Plant Genomic DNA Miniprep Kit. Purified genomic DNA (0.4 μg/lane) was analyzed on a 0.8% agarose gel. Marker is lambda Hind III digest.
Troubleshooting Guide |
|---|
Precautions and Disclaimer
The GenElute Plant Genomic DNA Miniprep Kit is for laboratory use only. Not for drug, household or other uses. The Lysis Solution [Part A] and the Binding Solution contain guanidine thiocyanate, which is harmful. The Column Preparation Solution is an irritant. Avoid contact with skin. Wear gloves, safety glasses, and suitable protective clothing when handling these solutions or any reagent provided with the kit. Please consult the Materials Safety Data Sheet (SDS) for information regarding hazards and safe handling practices.
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?