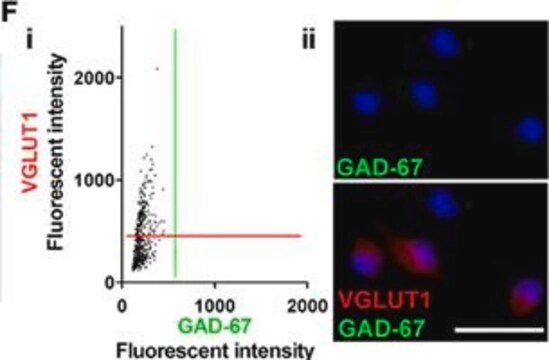
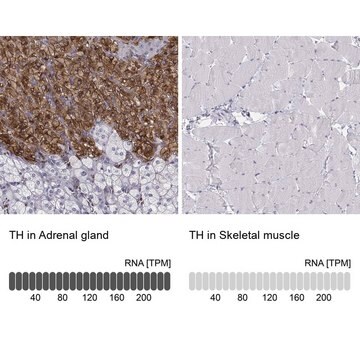
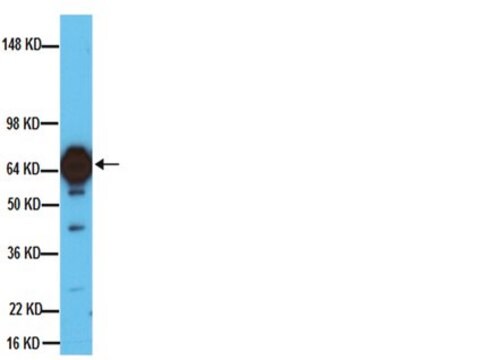
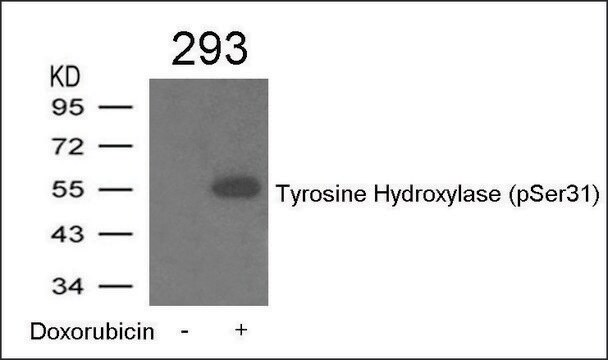
T2928
Monoclonal Anti-Tyrosine Hydroxylase antibody produced in mouse
clone TH-16, ascites fluid
Synonyme(s) :
Anti-DYT14, Anti-DYT5b, Anti-TYH
About This Item
Produits recommandés
Source biologique
mouse
Niveau de qualité
Conjugué
unconjugated
Forme d'anticorps
ascites fluid
Type de produit anticorps
primary antibodies
Clone
TH-16, monoclonal
Contient
15 mM sodium azide
Espèces réactives
rat, sheep, human, monkey, bovine, rabbit, guinea pig
Technique(s)
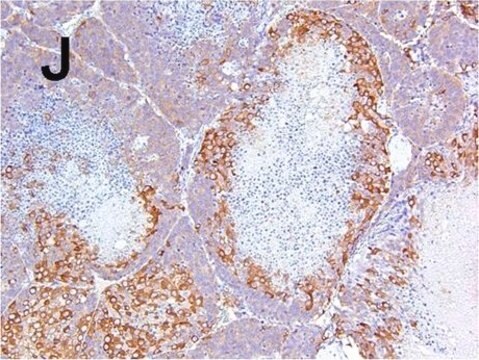
immunohistochemistry: suitable
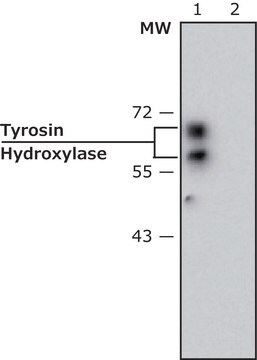
immunoprecipitation (IP): suitable using native or SDS-denatured TH.
microarray: suitable
western blot: 1:8,000 using extracts of cultured rat adrenal pheochromocytoma (PC12) cells.
Isotype
IgG1
Numéro d'accès UniProt
Conditions d'expédition
dry ice
Température de stockage
−20°C
Modification post-traductionnelle de la cible
unmodified
Description générale
Spécificité
Immunogène
Application
- western blotting
- immunofluorescence labelling
- immunohistochemical staining
- enzyme linked immunosorbent assay (ELISA)
- dot blot
- immunoprecipitation
- immunocytochemistry
Actions biochimiques/physiologiques
Clause de non-responsabilité
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
En option
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique