SBR00016
Aminopterin Ready Made Solution
50 mg/mL in DMSO
Synonyme(s) :
(S)-2-{4-[(2,4-Diaminopteridin-6-yl)methylamino]benzamido}pentanedioic acid, 4-Amino-PGA, 4-Aminofolic acid, 4-Aminopteroyl-L-glutamic acid
About This Item
Produits recommandés
Pureté
≥85%
Niveau de qualité
Forme
liquid
Conditions de stockage
protect from light
Concentration
50 mg/mL in DMSO
Solubilité
DMSO: 50 mg/mL
Conditions d'expédition
dry ice
Température de stockage
−20°C
Chaîne SMILES
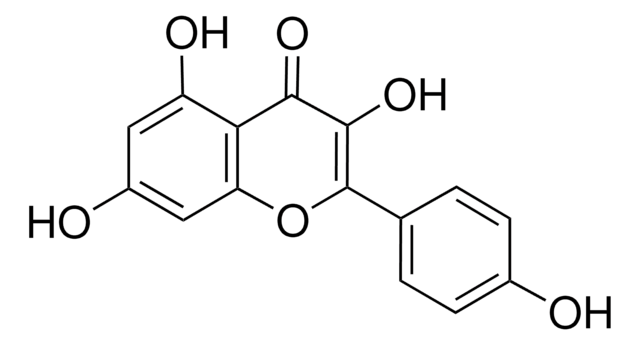
OC(CC[C@@H](C(O)=O)NC(C1=CC=C(NCC2=NC3=C(N)N=C(N)N=C3N=C2)C=C1)=O)=O
InChI
1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)
Clé InChI
TVZGACDUOSZQKY-UHFFFAOYSA-N
Actions biochimiques/physiologiques
Aminopterin is actively transported into cells by the folate transporter. In the cell, it is converted to a high molecular weight polyglutamate metabolite by folylpolyglutamate synthase that, in turn, binds to dihydrofolate reductase and inhibits its activity. Aminopterin-polyglutamate is degraded intracellularly by γ-glutamyl hydrolase.
Aminopterin was first administered for cancer therapy, as a drug targeting metabolism, specifically in pediatric leukemia. Later on it was demonstrated to be more potent yet more toxic than methotrexate.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Oral - Muta. 2 - Repr. 1B
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
188.6 °F
Point d'éclair (°C)
87 °C
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
This issue of Biofiles reviews some of our newest and most innovative technologies and their specific applications toward cancer research. In preparing this issue of Biofiles, one is reminded how complex the disease of cancer is, and how difficult it is to identify one topic that is completely unrelated to any other.
This article reviews some of our newest and most innovative technologies and their specific applications toward cancer research. It describes how complex the disease of cancer is, and how difficult it is to identify one topic that is completely unrelated to any other.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique