S9076
Superoxide Dismutase I human
recombinant, expressed in E. coli
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Source biologique
human
Niveau de qualité
Produit recombinant
expressed in E. coli
Essai
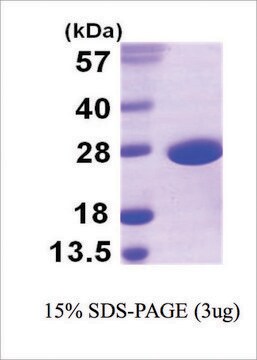
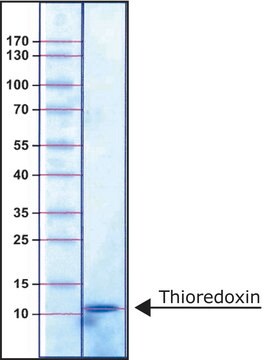
≥95% (SDS-PAGE and HPLC)
Forme
lyophilized powder
Poids mol.
31 kDa
Technique(s)
cell culture | mammalian: suitable
Couleur
white
Application(s)
detection
Conditions d'expédition
wet ice
Température de stockage
−20°C
Informations sur le gène
human ... SOD1(6647)
Description générale
Research Area: Cell Signaling
Cu/Zn superoxide dismutase (SOD1) is an intracellular antioxidant enzyme. A mature, functional human SOD1 is a relatively small (32 kDa) homodimeric metalloprotein.
Cu/Zn superoxide dismutase (SOD1) is an intracellular antioxidant enzyme. A mature, functional human SOD1 is a relatively small (32 kDa) homodimeric metalloprotein.
Application
Superoxide Dismutase I human has been used to treat THP-1 (human leukemia monocytic cell line) or human primary macrophage cells to confirm signal specificity for superoxide.
Actions biochimiques/physiologiques
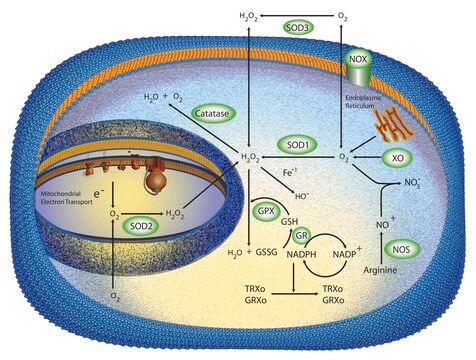
Cu/Zn superoxide dismutase (SOD1) regulates basal levels of oxidative stress arising from the production of mitochondrial and cytosolic superoxide (O2 .−). Its high cytosolic abundance makes it unique from the other two human superoxide dismutases. SOD1 was believed to be a copper (Cu) storage protein, however, the crucial role of SOD1 is to act as an intracellular antioxidant. SOD1 also initiates gene transcription following exposure to neurotoxic stimuli and modulates signal transduction pathways involving reactive oxygen species (ROS). However, it is also implicated in multiple molecular mechanisms of cytotoxicity, contributing to pathology in diseases such as heart failure, cancer, diabetes, Down′s syndrome, amyotrophic lateral sclerosis (ALS), and Parkinson′s disease.
Propriétés physiques
Recombinant Superoxide Dismutase I is fully biologically active when compared to standard.
Reconstitution
Reconstitute in H2O to a concentration of ≥100 μg/ml. The solution can then be diluted into other aqueous buffers.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Resp. Sens. 1
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Superoxide dismutases.
I Fridovich
Annual review of biochemistry, 44, 147-159 (1975-01-01)
J S Pollock et al.
Proceedings of the National Academy of Sciences of the United States of America, 88(23), 10480-10484 (1991-12-01)
The particulate enzyme responsible for the synthesis of endothelium-derived relaxing factor has been purified from cultured and native (noncultured) bovine aortic endothelial cells. Purification of the solubilized particulate enzyme preparation by affinity chromatography on adenosine 2',5'-bisphosphate coupled to Sepharose followed
S R Jolly et al.
Circulation research, 54(3), 277-285 (1984-03-01)
Therapy directed against the toxic effects of reactive oxygen species may reduce the final extent of ischemic injury in otherwise viable tissue irreversibly injured by the abrupt reoxygenation of reperfusion. In four groups of dogs, superoxide dismutase plus catalase (groups
S J Collins et al.
The Journal of experimental medicine, 149(4), 969-974 (1979-04-01)
The HL-60 human promyelocytic leukemia cell line can be induced to terminally differentiate to mature myeloid cells sharing a number of functional characteristics with normal granulocytes including response to chemoattractants, development of complement receptors, phagocytosis, superoxide production, and nitroblue tetrazolium
Ou-Yang Chao et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 68(1-2), 60-69 (2013-05-11)
We report cDNA cloning, expression, purification, and characterization of a novel Cu/ Zn superoxide dismutase (SOD) from Jatropha curcas leaves. The full-length cDNA of this SOD contained a 496-bp open-reading frame (ORF) encoding 162 amino acid residues. The recombinant plasmid
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique