P4110
Monoclonal Anti-Phosphotyrosine antibody produced in mouse
clone PY20, purified immunoglobulin, buffered aqueous glycerol solution
Synonyme(s) :
Monoclonal Anti-Phosphotyrosine, Phospho-Tyr, Phospho-tyrosine, p-Tyr
About This Item
Produits recommandés
Source biologique
mouse
Niveau de qualité
Conjugué
unconjugated
Forme d'anticorps
purified immunoglobulin
Type de produit anticorps
primary antibodies
Clone
PY20, monoclonal
Forme
buffered aqueous glycerol solution
Technique(s)
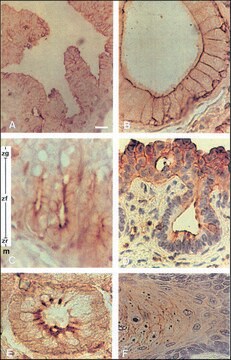
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
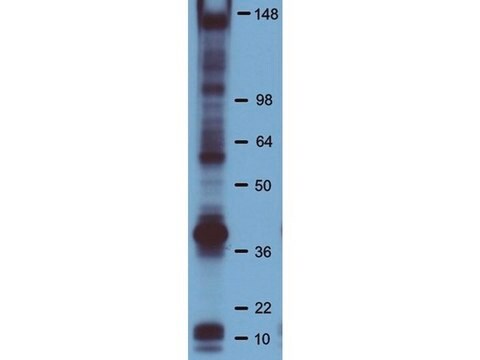
western blot: suitable
Isotype
IgG2b
Conditions d'expédition
wet ice
Température de stockage
−20°C
Modification post-traductionnelle de la cible
phosphorylation (pTyr)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
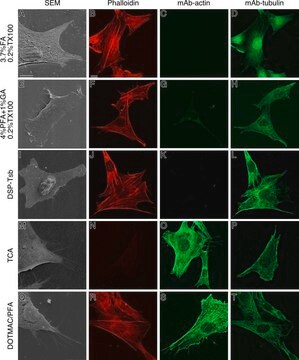
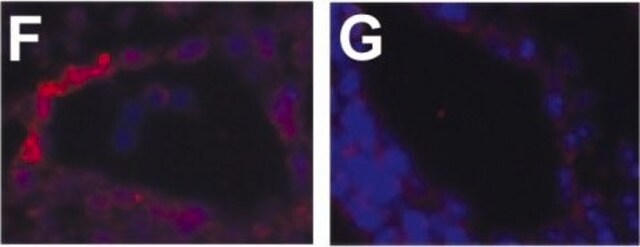
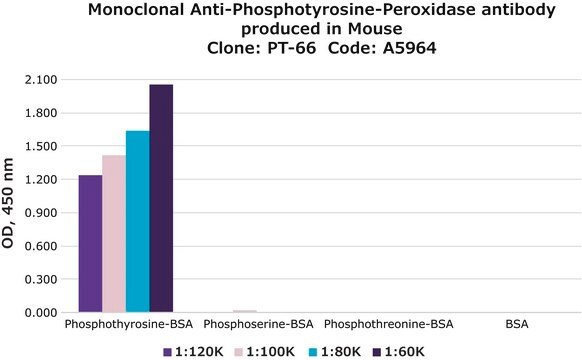
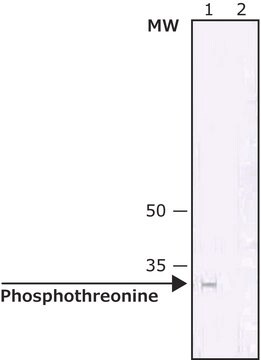
Description générale
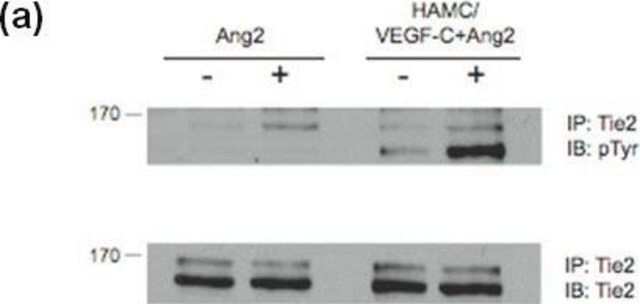
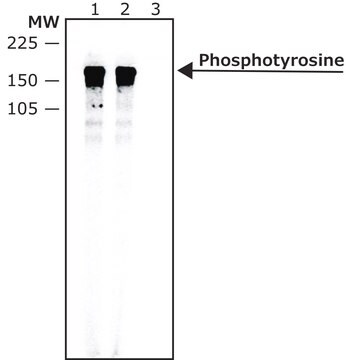
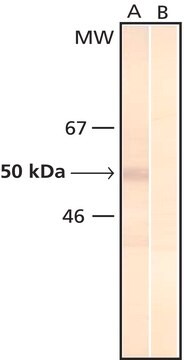
Monoclonal Anti-Phosphotyrosine is specific for both native and denatured proteins containing phosphorylated tyrosine.
Immunogène
Application
Forme physique
Clause de non-responsabilité
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
nwg
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique