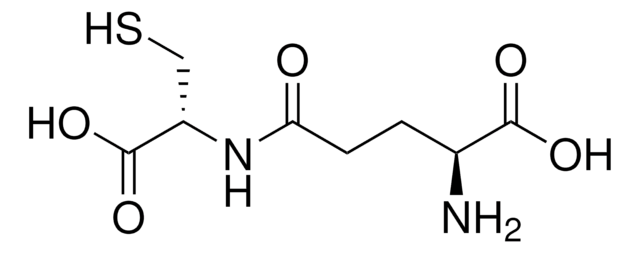
M6626
S-Methyl-L-cysteine
suitable for ligand binding assays
Synonyme(s) :
(R)-2-Amino-3-(methylmercapto)propionic acid, SMLC
About This Item
Produits recommandés
product name
S-Methyl-L-cysteine, substrate for methionine sulfoxide reductase A
Niveau de qualité
Forme
powder
Technique(s)
ligand binding assay: suitable
Application(s)
peptide synthesis
Température de stockage
−20°C
Chaîne SMILES
CSC[C@H](N)C(O)=O
InChI
1S/C4H9NO2S/c1-8-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m0/s1
Clé InChI
IDIDJDIHTAOVLG-VKHMYHEASA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- In-vitro antioxidant and anti-inflammatory potential along with p.o. pharmacokinetic profile of key bioactive phytocompounds of Snow Mountain Garlic: a comparative analysis vis-à-vis normal garlic.: This study examines the antioxidant and anti-inflammatory properties of S-Methyl-L-cysteine found in Snow Mountain Garlic, providing insights into its potential therapeutic applications (Kaur et al., 2024).
- Acidification and tissue disruption affect glucosinolate and S-methyl-l-cysteine sulfoxide hydrolysis and formation of amines, isothiocyanates and other organosulfur compounds in red cabbage (Brassica oleracea var. capitata f. rubra).: The research highlights how acidification and tissue disruption influence the hydrolysis of S-Methyl-L-cysteine sulfoxide, impacting the formation of various bioactive compounds in red cabbage (Hanschen, 2024).
- Selective cycloaddition of ethylene oxide to CO(2) within the confined space of an amino acid-based metal-organic framework.: This research explores the use of an S-Methyl-L-cysteine-based metal-organic framework for the selective cycloaddition of ethylene oxide to CO2, demonstrating its potential in green chemistry and materials science (Bilanin et al., 2023).
Actions biochimiques/physiologiques
When used as a dietary supplement in the Drosphilia model of PD, SMLC increases the efficacy of the MRSA catalytic antioxidant system by providing additional substrate available leading to increased resistance to oxidative stress.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique