L5135
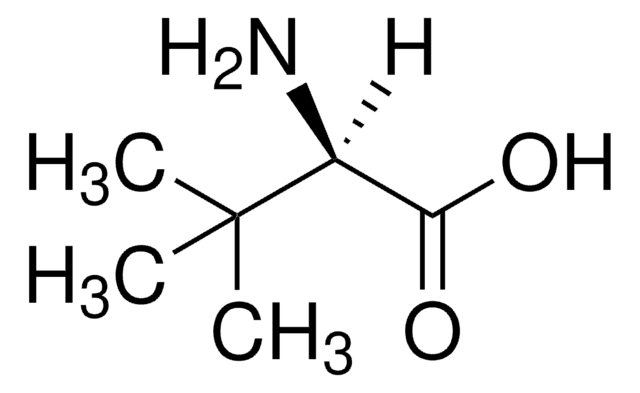
L-Leucine Dehydrogenase from Bacillus cereus
lyophilized powder, ≥60 units/mg protein
Synonyme(s) :
L-Leucine:NAD+ oxidoreductase (deaminating)
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Numéro CAS:
Numéro MDL:
Code UNSPSC :
12352204
Nomenclature NACRES :
NA.54
Produits recommandés
Forme
lyophilized powder
Niveau de qualité
Activité spécifique
≥60 units/mg protein
Poids mol.
245 kDa
Température de stockage
−20°C
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
L-Leucine Dehydrogenase is a member of the amino acid dehydrogenase family.
Application
L-Leucine Dehydrogenase from Bacillus cereus has been used to determine the branched-chain amino acids (BCAA) spectrophotometrically in serum samples.
Actions biochimiques/physiologiques
Leucine Dehydrogenase is a nicotinamide adenine dinucleotide hydrogen (NADH)-dependent oxidoreductase. It is involved in catalyzing the reductive amination of aliphatic 2-oxo-acids to their respective L-amino acids.
Définition de l'unité
One unit will convert 1.0 μmole of L‑leucine to α-ketoisocaproate per min at pH 10.5 at 37 °C.
Autres remarques
contains lysine
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
K Selber et al.
Journal of chromatography. B, Biomedical sciences and applications, 743(1-2), 21-30 (2000-08-15)
Mathematical strategies were applied to optimise the extraction of recombinant leucine dehydrogenase from E. coli homogenates and endoglucanase 1 from culture filtrates of Trichoderma reesei in polyethylene glycol-phosphate systems. The goal was to test mathematical tools which could facilitate the
M B Ansorge et al.
Applied microbiology and biotechnology, 53(6), 668-673 (2000-08-05)
The established Escherichia coli expression vectors ptrc99a, pKK223-3, pPLlambda, pAsk75, pRA95, and pRA96, which differ in copy number, mode of induction, selection marker, and use of par sequences for stabilization, were investigated for the stable expression of recombinant L-leucine dehydrogenase
S Guangdong et al.
The Journal of antibiotics, 54(1), 66-73 (2001-03-28)
Shengjimycin is a group of 4"-acylated spiramycins with 4"-isovalerylspiramycin as the major component, produced by recombinant S. spiramyceticus F21 harboring a 4"-O-acyltransferase gene from S. mycarofaciens 1748. A stable bioengineered strain of Streptomyces spiramyceticus WSJ-1 was constructed by integrating the
Peng-Hu Zhang et al.
Sheng wu gong cheng xue bao = Chinese journal of biotechnology, 23(2), 268-272 (2007-04-28)
The purification and the characteristics of an enzyme from Morganella morganii J-8, which could produce d-pseudoephedrine from 1-phenyl-2-methylamine-acetone, were performed in this study. In this research, first, cells were disrupted by ultrasonic treatment at 4 degrees C. The carbonyl enantioselective
Tatyana A Muranova et al.
Acta crystallographica. Section D, Biological crystallography, 58(Pt 6 Pt 2), 1059-1062 (2002-05-31)
Leucine dehydrogenase is an octameric enzyme which belongs to the superfamily of amino-acid dehydrogenases and catalyses the reversible oxidative deamination of leucine to 2-ketoisocaproate, with the corresponding reduction of the cofactor NAD(+). Catalysis by this enzyme is thought to involve
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique