K3502
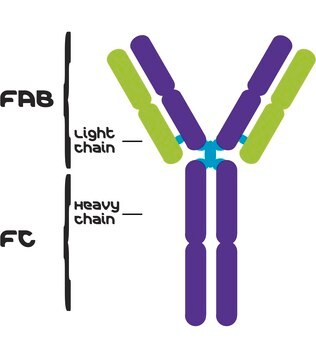
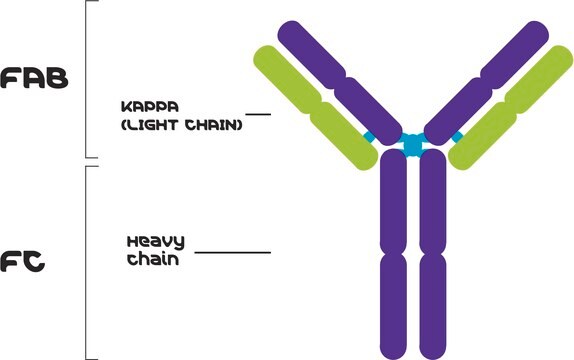
Anti-Human Kappa Light Chain (Bound and Free) antibody produced in goat
affinity isolated antibody, lyophilized powder
About This Item
Produits recommandés
Source biologique
goat
Niveau de qualité
Conjugué
unconjugated
Forme d'anticorps
affinity isolated antibody
Type de produit anticorps
secondary antibodies
Clone
polyclonal
Forme
lyophilized powder
Technique(s)
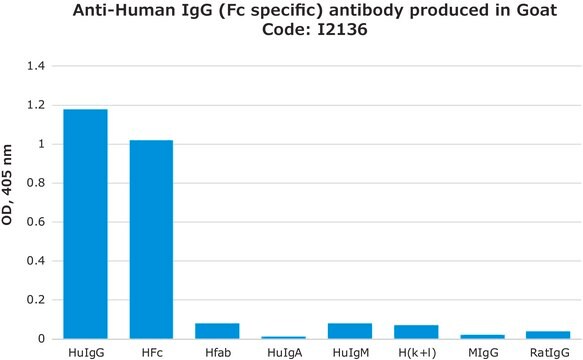
quantitative precipitin assay: suitable
Température de stockage
2-8°C
Modification post-traductionnelle de la cible
unmodified
Informations sur le gène
human ... IGK@(50802)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Immunogène
Application
Forme physique
Reconstitution
Clause de non-responsabilité
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique