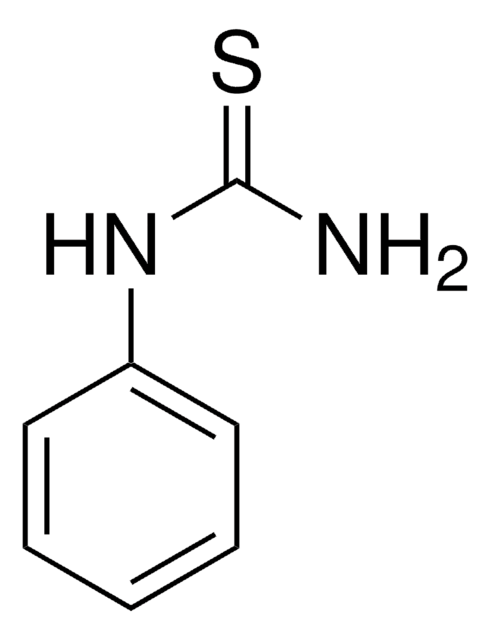
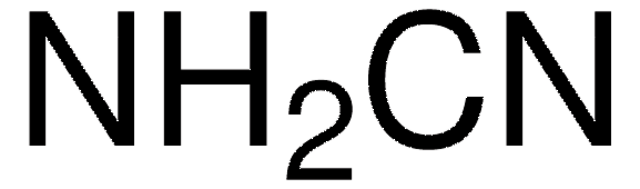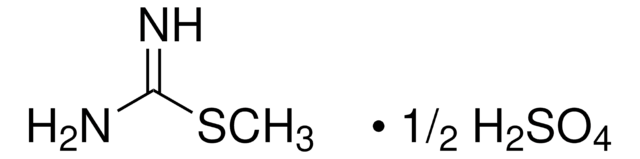H7654
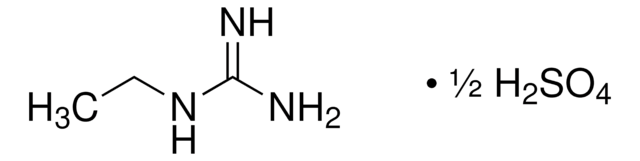
Hydroxyguanidine sulfate salt
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
CH5N3O · 1/2H2SO4
Numéro CAS:
Poids moléculaire :
124.11
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352202
ID de substance PubChem :
Nomenclature NACRES :
NA.77
Produits recommandés
Source biologique
synthetic (organic)
Essai
≥98% (TLC)
Forme
powder
Solubilité
water: 25 mg/mL, clear, colorless
Température de stockage
2-8°C
Chaîne SMILES
NC(=N)NO.NC(=N)NO.OS(O)(=O)=O
InChI
1S/2CH5N3O.H2O4S/c2*2-1(3)4-5;1-5(2,3)4/h2*5H,(H4,2,3,4);(H2,1,2,3,4)
Clé InChI
MTGDDPZRXSDPFH-UHFFFAOYSA-N
Actions biochimiques/physiologiques
An early antitumor agent. Oxidation results in release of NO, and formation of other reactive oxygen species, including peroxynitrite and peroxyl radicals. Reacts with NO to form an adduct which is a potent and stable vasodilator.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Rémy Ricoux et al.
European journal of biochemistry, 270(1), 47-55 (2002-12-21)
Nitric oxide (NO) is a potent intra- and intercellular messenger involved in the control of vascular tone, neuronal signalling and host response to infection. In mammals, NO is synthesized by oxidation of l-arginine catalysed by hemeproteins called NO-synthases with intermediate
Ming Xian et al.
Bioorganic & medicinal chemistry, 10(9), 3049-3055 (2002-07-12)
Enzymatic generation of nitric oxide (NO) by nitric oxide synthase (NOS) consists of two oxidation steps. The first step converts L-arginine to N(G)-hydroxy-L-arginine (NOHA), a key intermediate, and the second step converts NOHA to NO and L-citrulline. To fully probe
Tingwei Cai et al.
Bioorganic & medicinal chemistry letters, 12(11), 1507-1510 (2002-05-29)
The electrochemical properties of a series of N-substituted-N'-hydroxyguanidines were studied. Two oxidation potentials of each compound were obtained by cyclic voltammetry. The E(ox1) values were from 0.51 to 0.62V, while the E(ox2) values were from 1.14 to 1.81V in acetonitrile
David Lefèvre-Groboillot et al.
Biochemistry, 42(13), 3858-3867 (2003-04-02)
The interaction of various N-alkyl- and N-aryl-N'-hydroxyguanidines with recombinant NOS containing or not containing tetrahydrobiopterin (BH(4)) was studied by visible, electronic paramagnetic resonance (EPR), and resonance Raman (RR) spectroscopy. N-Hydroxyguanidines interact with the oxygenase domain of BH(4)-free inducible NOS (BH(4)-free
S A Everett et al.
Free radical biology & medicine, 24(1), 1-10 (1998-01-22)
The oxidative denitrification of the antitumour agent hydroxyguanidine (HOG) has been investigated by radiolysis methods and EPR spectroscopy. The azide radical (N3.), a model one-electron oxidant, reacts with HOG with the rate constant 5.1 x 10(9) dm3 mol(-1) s(-1) to
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique