H6660
HIV Protease Substrate 1
≥95% (HPLC), powder
Synonyme(s) :
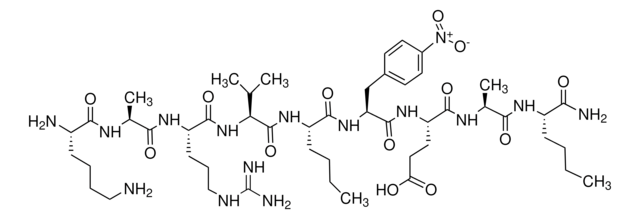
Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Nom du produit
HIV Protease Substrate 1,
Essai
≥95% (HPLC)
Niveau de qualité
Forme
powder
Poids mol.
2016
Solubilité
DMSO: soluble
Température de stockage
−20°C
Application
HIV Protease Substrate 1 has been used:
- to study protein structure-function relationships and in the fluorescent assay
- to evaluate protease inhibitors and for in vitro detection of the HIV-1 protease activity
- and in protease kinetics to study its enzymatic degradation rates
Actions biochimiques/physiologiques
HIV Protease Substrate 1 is a synthetic peptide sequence that contains the cleavage site (Tyr-Pro) for the human immunodeficiency virus (HIV) Protease. It has two covalently modified amino acids for the detection of cleavage. One modification is the addition of the fluorophore 5-(2-aminoethylamino)-1-naphthalene sulfonate (EDANS) to the glutamic acid residue, while the other one includes the addition of the acceptor chromophore 4c-dimethylaminoazobenzene-4-carboxylate (DABCYL) to the lysine residue. The modified amino acids are on opposite sides of the cleavage site. Spatial orientation and overlap of the DABCYL absorbance with the EDANS emission permit resonance energy transfer between the two moieties. However, when the peptide is cleaved by the HIV protease, the DABCYL group is no longer proximal to the fluorophore.
Substrate for endopeptidases
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Sagheer A Onaizi et al.
Langmuir : the ACS journal of surfaces and colloids, 23(11), 6336-6341 (2007-04-24)
We present the first study of the directed disassembly of a protein network at the air-water interface by the synergistic action of a surfactant and an enzyme. We seek to understand the fundamentals of protein network disassembly by using rubisco
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique