H4645
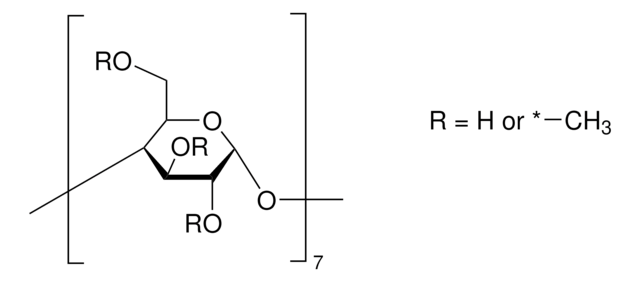
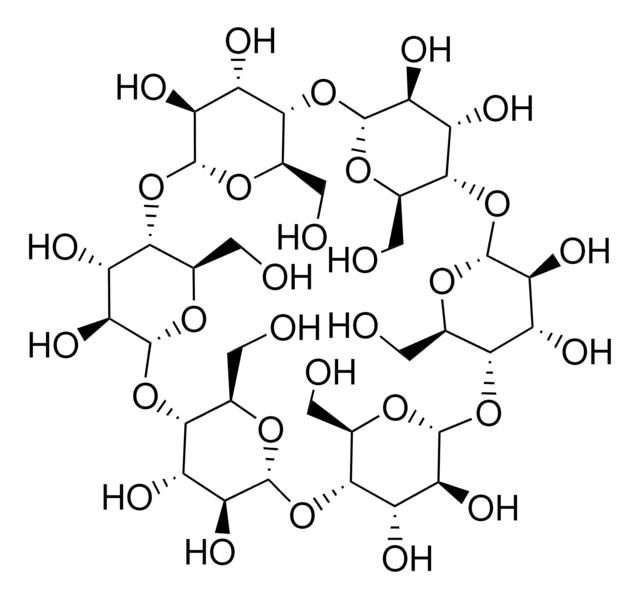
Heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin
≥90%
Synonyme(s) :
2,3,6-Tri-O-methyl-β-cyclodextrin, Trimethyl-β-cyclodextrin
About This Item
Produits recommandés
Niveau de qualité
Essai
≥90%
Forme
powder
Technique(s)
electrophoresis: suitable
Pf
170-178 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
COC[C@H]1O[C@@H]2O[C@@H]3[C@@H](COC)O[C@H](O[C@@H]4[C@@H](COC)O[C@H](O[C@@H]5[C@@H](COC)O[C@H](O[C@@H]6[C@@H](COC)O[C@H](O[C@@H]7[C@@H](COC)O[C@H](O[C@@H]8[C@@H](COC)O[C@H](O[C@H]1[C@H](OC)[C@H]2OC)[C@H](OC)[C@H]8OC)[C@H](OC)[C@H]7OC)[C@H](OC)[C@H]6OC)[C@H](OC)[C@H]5OC)[C@H](OC)[C@H]4OC)[C@H](OC)[C@H]3OC
InChI
1S/C63H112O35/c1-64-22-29-36-43(71-8)50(78-15)57(85-29)93-37-30(23-65-2)87-59(52(80-17)44(37)72-9)95-39-32(25-67-4)89-61(54(82-19)46(39)74-11)97-41-34(27-69-6)91-63(56(84-21)48(41)76-13)98-42-35(28-70-7)90-62(55(83-20)49(42)77-14)96-40-33(26-68-5)88-60(53(81-18)47(40)75-12)94-38-31(24-66-3)86-58(92-36)51(79-16)45(38)73-10/h29-63H,22-28H2,1-21H3/t29-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43+,44+,45+,46+,47+,48+,49+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-/m1/s1
Clé InChI
DSDAICPXUXPBCC-MWDJDSKUSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- To investigate the crystal structure of its complexes with m-iodophenol, 4-biphenylacetic acid and (R)- and (S)-flurbiprofen by X-ray analysis.
- To study the candidature of its complex with vitamin A for potential application as a drug delivery system for ophthalmic applications by high sensitivity fluorescence spectrometry and high pressure liquid chromatography (HPLC) techniques.
- In the determination of the analyte composition in commercial samples by HPLC coupled to mass spectrometry (MS).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique