G1763
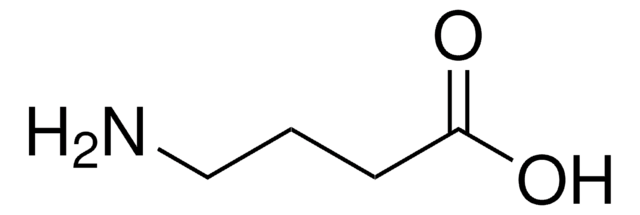
β-Glutamic acid
≥98% (TLC), suitable for ligand binding assays
Synonyme(s) :
3-Aminopentanedioic acid
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C5H9NO4
Numéro CAS:
Poids moléculaire :
147.13
Numéro MDL:
Code UNSPSC :
12352209
ID de substance PubChem :
Nomenclature NACRES :
NA.26
Produits recommandés
Nom du produit
β-Glutamic acid,
Essai
≥98% (TLC)
Niveau de qualité
Forme
powder
Technique(s)
ligand binding assay: suitable
Couleur
white to off-white
Chaîne SMILES
NC(CC(O)=O)CC(O)=O
InChI
1S/C5H9NO4/c6-3(1-4(7)8)2-5(9)10/h3H,1-2,6H2,(H,7,8)(H,9,10)
Clé InChI
BBJIPMIXTXKYLZ-UHFFFAOYSA-N
Actions biochimiques/physiologiques
β-Glutamic acid (β-Glu) is used as an osmolyte in many archaea. β-Glutamic acid may be used as a substrate to study the specificity and kinetics of archaeal and bacterial glutamine synthetase (GS) enzymes. β-Glutamic acid is used to study primitive mechanisms of polypeptide formation.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
H McLennan et al.
Neuropharmacology, 21(6), 549-554 (1982-06-01)
Separate receptors are recognized for the excitation of mammalian neurones by (a) L-glutamic and quisqualic acids and (b) N-methyl-D-aspartic (NMDA), and other amino acids which have conformationally restricted molecules. Several other compounds, both agonists and antagonists, have been examined, and
Jean-François Lambert
Origins of life and evolution of the biosphere : the journal of the International Society for the Study of the Origin of Life, 38(3), 211-242 (2008-03-18)
The present paper offers a review of recent (post-1980) work on amino acid adsorption and thermal reactivity on oxide and sulfide minerals. This review is performed in the general frame of evaluating Bernal's hypothesis of prebiotic polymerization in the adsorbed
D E Robertson et al.
Biochimica et biophysica acta, 992(3), 320-326 (1989-09-15)
13C- and 15N-NMR spectroscopy have been used to identify beta-aminoglutaric acid (beta-glutamic) as a major soluble component of the thermophilic, autotrophic marine methanogen Methanococcus thermolithotrophicus. This rare, non-protein amino acid has been recognized as a major dissolved free amino acid
P Robinson et al.
Applied and environmental microbiology, 67(10), 4458-4463 (2001-09-26)
The conversion of beta-glutamate to beta-glutamine by archaeal and bacterial glutamine synthetase (GS) enzymes has been examined. The GS from Methanohalophilus portucalensis (which was partially purified) is capable of catalyzing the amidation of this substrate with a rate sevenfold less
D E Robertson et al.
Applied and environmental microbiology, 56(5), 1504-1508 (1990-05-01)
The unusual compound beta-aminoglutaric acid (beta-glutamate) has been identified by 13C nuclear magnetic resonance spectroscopy in soluble extracts of marine methanogenic bacteria. We examined several methanogen species representing nine genera and found that beta-glutamate occurred in methanococci and two methanogenium
Chromatograms
application for HPLCNotre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique