E3763
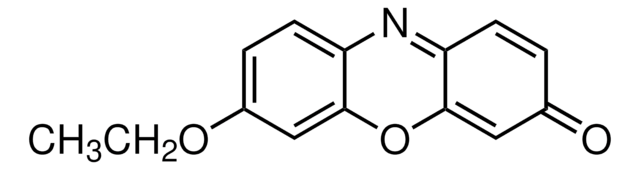
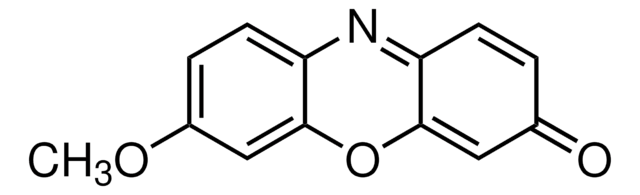
Resorufin ethyl ether
≥98% (TLC), powder
Synonyme(s) :
7-Ethoxy-3H-phenoxazin-3-one, Ethoxyresorufin, O7-Ethylresorufin
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥98% (TLC)
Forme
powder
Conditions de stockage
(Tightly closed. Dry)
Technique(s)
activity assay: suitable
Couleur
orange to red
Pf
223-225 °C (lit.)
Solubilité
chloroform: 9.80-10.20 mg/mL, clear, orange
Adéquation
suitable for fluorescence
Température de stockage
−20°C
Chaîne SMILES
CCOc1ccc2N=C3C=CC(=O)C=C3Oc2c1.Fc4c(F)c(F)c(OC(=O)CNC(=O)OCC5c6ccccc6-c7ccccc57)c(F)c4F
InChI
1S/C23H14F5NO4.C14H11NO3/c24-17-18(25)20(27)22(21(28)19(17)26)33-16(30)9-29-23(31)32-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15;1-2-17-10-4-6-12-14(8-10)18-13-7-9(16)3-5-11(13)15-12/h1-8,15H,9-10H2,(H,29,31);3-8H,2H2,1H3
Clé InChI
ZOSYTBPPLWBBKM-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Research area: Cell Signaling
Application
Substrats
Substrat
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Phase I biotransformation reactions introduce or expose functional groups on the drug with the goal of increasing the polarity of the compound. Although Phase I drug metabolism occurs in most tissues, the primary and first pass site of metabolism occurs during hepatic circulation.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique