D5782
2′,3′-Dideoxycytidine
≥98% (HPLC)
Synonyme(s) :
ddC
About This Item
Produits recommandés
Source biologique
synthetic (organic)
Essai
≥98% (HPLC)
Forme
powder
Couleur
colorless
Pf
217-218 °C (lit.)
Solubilité
water: 50 mg/mL, clear, colorless to faintly yellow
Température de stockage
−20°C
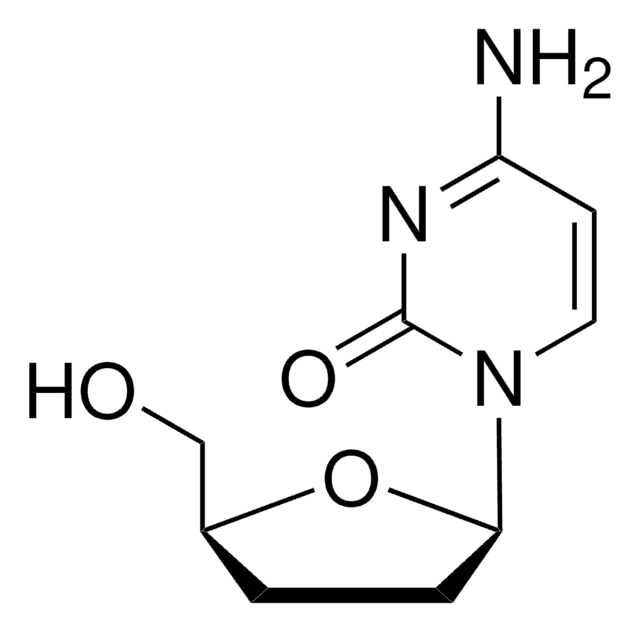
Chaîne SMILES
NC1=NC(=O)N(C=C1)[C@H]2CC[C@@H](CO)O2
InChI
1S/C9H13N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h3-4,6,8,13H,1-2,5H2,(H2,10,11,14)/t6-,8+/m0/s1
Clé InChI
WREGKURFCTUGRC-POYBYMJQSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- as a DNA chain-terminating nucleotide for DNA sequencing methods based on the Sanger chain-termination method
- as a nucleoside reverse transcriptase inhibitor (NRTI) to study its effects on the development of mechanical allodynia in aging mice
- as a mitochondrial DNA (mtDNA) replication inhibitor to inhibit the activation of cGAS-STING pathway and study its effects on signaling protein-stimulator of interferon genes (STING), cyclic GMP-AMP synthase (cGAS), and phospho-interferon regulator factor 3 (p-IRF3) expression in mouse hippocampal and microglial cells
- as an NRTI inhibitor to study its effects on the drug induced-mitochondrial toxicity in Caenorhabditis elegans
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Carc. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique