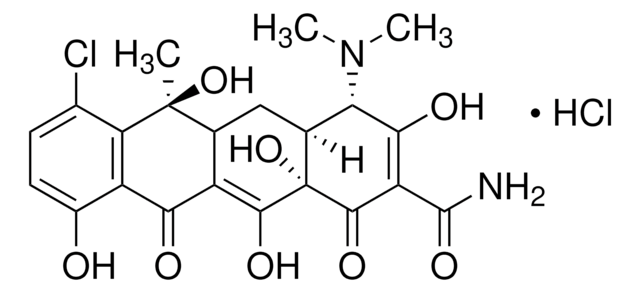
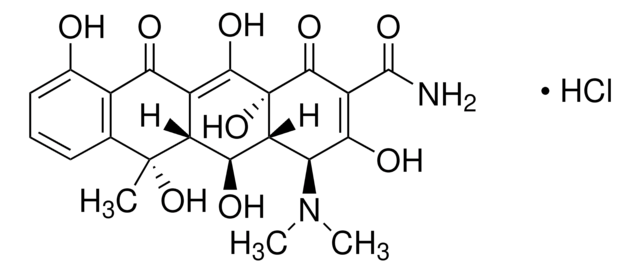
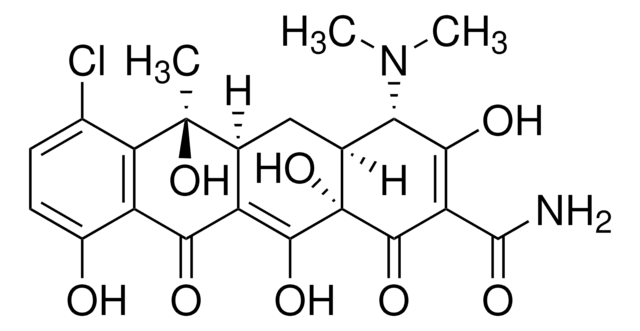
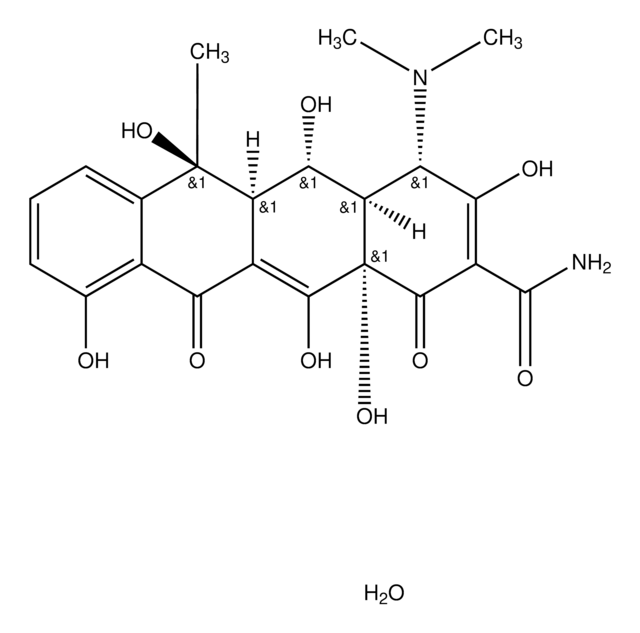
C4881
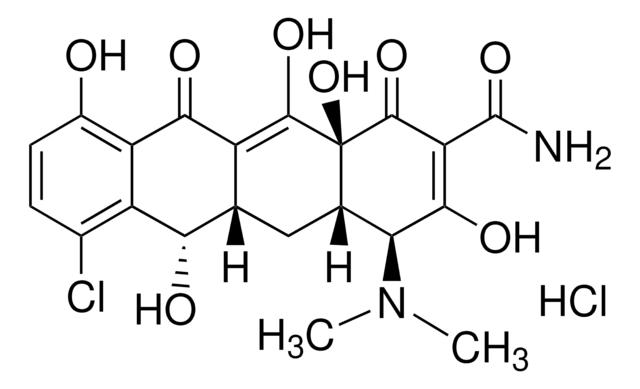
Chlortetracycline hydrochloride
≥91.0% dry basis (HPLC)
Synonyme(s) :
7-Chlorotetracycline hydrochloride
About This Item
Produits recommandés
Niveau de qualité
Essai
≥91.0% dry basis (HPLC)
Forme
powder
pKa (25 °C)
3.3
7.4
9.3
Pf
210-215 °C (dec.) (lit.)
Solubilité
1 M NaOH: 50 mg/mL
Spectre d'activité de l'antibiotique
Gram-negative bacteria
Gram-positive bacteria
Mode d’action
protein synthesis | interferes
Température de stockage
−20°C
Chaîne SMILES
Cl.CN(C)[C@H]1[C@@H]2CC3C(=C(O)[C@]2(O)C(=O)C(C(N)=O)=C1O)C(=O)c4c(O)ccc(Cl)c4[C@@]3(C)O
InChI
1S/C22H23ClN2O8.ClH/c1-21(32)7-6-8-15(25(2)3)17(28)13(20(24)31)19(30)22(8,33)18(29)11(7)16(27)12-10(26)5-4-9(23)14(12)21;/h4-5,7-8,15,26,28-29,32-33H,6H2,1-3H3,(H2,24,31);1H/t7?,8-,15-,21-,22-;/m0./s1
Clé InChI
CBHYYLPALVVVEY-LYNLVHCPSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
Actions biochimiques/physiologiques
Mode of Resistance: Loss of cell wall permeability.
Autres remarques
Produit(s) apparenté(s)
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique