C0400
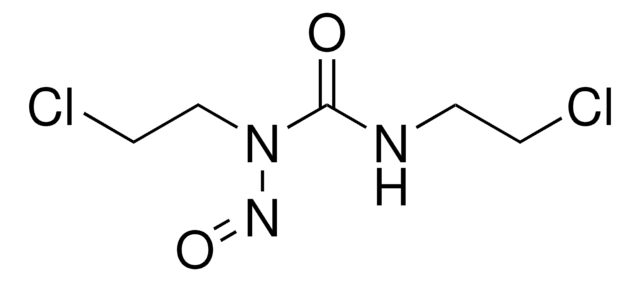
Carmustine
≥98% (TLC), oily liquid to amorphous solid, DNA alkylating agent
Synonyme(s) :
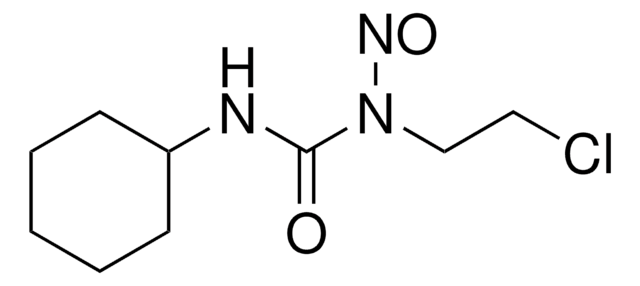
1,3-Bis(2-chloroethyl)-1-nitrosourea, BCNU
About This Item
Produits recommandés
Nom du produit
Carmustine, ≥98%
Niveau de qualité
Essai
≥98%
Forme
(Oily liquid to amorphous solid)
Pf
30 °C (lit.)
Solubilité
ethanol: 19.60-20.40 mg/mL, clear, pale yellow to yellow
Auteur
Bristol-Myers Squibb
Température de stockage
−20°C
Chaîne SMILES
ClCCNC(=O)N(CCCl)N=O
InChI
1S/C5H9Cl2N3O2/c6-1-3-8-5(11)10(9-12)4-2-7/h1-4H2,(H,8,11)
Clé InChI
DLGOEMSEDOSKAD-UHFFFAOYSA-N
Informations sur le gène
human ... GSR(2936)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
Caractéristiques et avantages
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 2 Oral - Carc. 1B - Repr. 1B
Code de la classe de stockage
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Contenu apparenté
Apoptosis, or programmed cell death (PCD), is a selective process for the removal of unnecessary, infected or transformed cells in various biological systems. As it plays a role in the homeostasis of multicellular organisms, apoptosis is tightly regulated through two principal pathways by a number of regulatory and effector molecules.
n proliferating cells, the cell cycle consists of four phases. Gap 1 (G1) is the interval between mitosis and DNA replication that is characterized by cell growth. Replication of DNA occurs during the synthesis (S) phase, which is followed by a second gap phase (G2) during which growth and preparation for cell division occurs. Together, these three stages comprise the interphase phase of the cell cycle. Interphase is followed by the mitotic (M) phase.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique