A8008
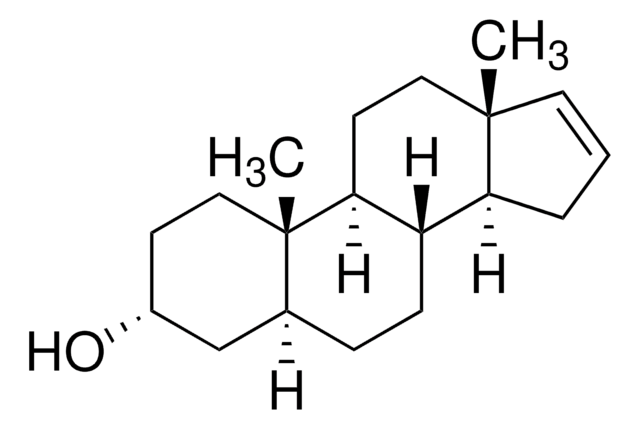
5α-Androst-16-en-3-one
Synonyme(s) :
16-(5α)Androsten-3-one, 3-Keto-5α,16-androstene, Androstenone
About This Item
Produits recommandés
Source biologique
synthetic (organic)
Niveau de qualité
Essai
≥98% (TLC)
Forme
powder
Pf
140-145 °C (lit.)
Solubilité
dichloromethane: 9.80-10.20 mg/mL, clear, colorless to faintly yellow
Conditions d'expédition
ambient
Température de stockage
room temp
Chaîne SMILES
[H][C@@]12CC[C@]3([H])[C@]([H])(CC[C@]4(C)C=CC[C@@]34[H])[C@@]1(C)CCC(=O)C2
InChI
1S/C19H28O/c1-18-9-3-4-16(18)15-6-5-13-12-14(20)7-11-19(13,2)17(15)8-10-18/h3,9,13,15-17H,4-8,10-12H2,1-2H3/t13-,15-,16-,17-,18-,19-/m0/s1
Clé InChI
HFVMLYAGWXSTQI-QYXZOKGRSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- to quantify its concentration in various fat tissues
- to study its trigeminal percept and implications on the rate of specific anosmia
- to study the effects of immunocastration on meat quality and sensory properties of pork bellies
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique