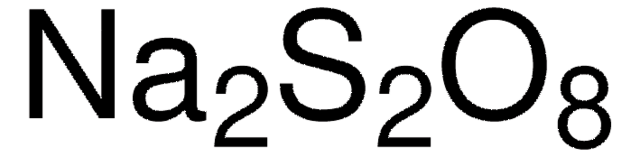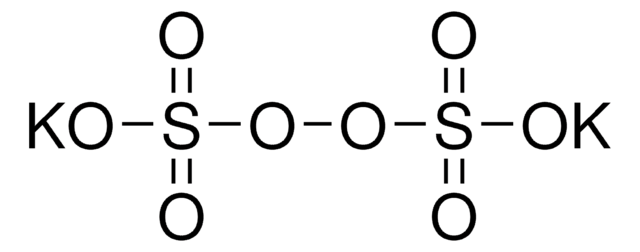216232
Sodium persulfate
reagent grade, ≥98%
Synonyme(s) :
Sodium peroxodisulfate
About This Item
Produits recommandés
Qualité
reagent grade
Niveau de qualité
Essai
≥98%
Forme
powder, crystals or granules
Pertinence de la réaction
reagent type: oxidant
Chaîne SMILES
[Na+].[Na+].[O-]S(=O)(=O)OOS([O-])(=O)=O
InChI
1S/2Na.H2O8S2/c;;1-9(2,3)7-8-10(4,5)6/h;;(H,1,2,3)(H,4,5,6)/q2*+1;/p-2
Clé InChI
CHQMHPLRPQMAMX-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- For the radical cyclization cascade reaction of 2-alkynylbenzonitriles and sodium arylsulfinates to synthesize sulfonated indenones.
- In the silica-supported aluminum chloride-catalyzed Baeyer-Villiger oxidation of cyclic and acyclic ketones to lactones or esters.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Ox. Sol. 3 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
5.1B - Oxidizing hazardous materials
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique